Protected: Soft Tissue Arrays


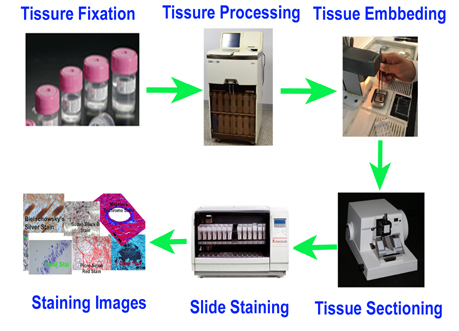
Routine histology services are essential for tissue-based research and diagnostics, enabling researchers and pathologists to analyze tissue morphology, identify specific cell types, and detect microorganisms like bacteria. Histological staining enhances tissue visualization by adding contrast to otherwise transparent tissue sections.
We offer a full range of histology lab services, from tissue processing to high-quality staining:
✅ Tissue Trimming – Precision trimming for optimal sectioning.
✅ Tissue Processing – Formalin fixation and paraffin embedding (FFPE).
✅ Paraffin Embedding – Long-term preservation of tissue samples.
✅ Tissue Sectioning – Thin section cutting from paraffin-embedded blocks.
✅ Slide Preparation – Mounting on positively charged or custom-treated slides.
✅ H&E & Special Staining – High-quality staining for enhanced tissue visualization.
🔬 Get Expert Routine Histology Services Today! Contact us for high-quality tissue processing, embedding, and staining solutions tailored to your research needs.
Hematoxylin and Eosin (H&E) stain is the most commonly used staining system. It is an important part of VitroVivo routine histology services. H&E contains two dyes haemotoxylin and eosin. Eosin is an acidic dye: it is negatively charged (general formula for acidic dyes is: Na+dye-). It stains basic (or acidophilic) structures red or pink. This is also sometimes termed ‘eosinophilic’. Thus the cytoplasm is stained pink by H&E staining.
Hematoxylin can be considered as a basic dye (general formula for basic dyes is:dye+ Cl-). Hematoxylin is actually a dye called hematein (obtained from the log-wood tree) used in combination with aluminium ions (Al3+). It is used to stain acidic (or basophilic) structures a purplish blue. (Hematoxylin is not strictly a basic dye, but it is used with a ‘mordant’ that makes this stain act as a basic dye. The mordant (aluminium salts) binds to the tissue, and then hematoxylin binds to the mordant, forming a tissue-mordant-hematoxylin linkage.) Thus the nucleus is stained purple by H&E staining.
This means that the nucleus, and parts of the cytoplasm that contain RNA stain up in one color (purple), and the rest of the cytoplasm stains up a different color (pink)
At VitroVivo, a leading histology and molecular histopathology company, we offer comprehensive FFPE (Formalin-Fixed Paraffin-Embedded), Frozen, and Laser Capture Microdissection (LCM) sample analysis services. Our expert team of biomedical scientists provides high-quality services to accelerate your research, including:
Our services are designed to streamline your research projects and enhance the accuracy of your molecular studies. For more information or to request services, please contact us at service@vitrovivo.com.

At VitroVivo, we provide expert histopathological interpretation and microscopic analysis by Board-Certified Pathologists. Our services include:
Our pathologists deliver precise and reliable histopathological interpretations to support your research, diagnostics, and publication needs. Contact us for professional and accurate pathological analysis services.
VitroVivo acceptes wet tissue, frozen samples, FFPE blocks, OCT blocks, unstained slides, stained slides for histology and pathology review or microscopic description. We also accept high quality whole slide microscope images. If necessary, we can perform the rest of the experiment for histopathology review.
Our pathologists review both human and animal tissue slides.
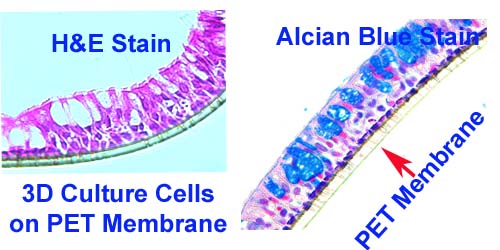
Any of several microscopic sections made and arranged in consecutive order are called serial section. A continuous series of sections reveal structures in three dimensions (3D).
Step sectioning means taking a section and putting it on a slide, then shaving into the tissue a set distance, perhaps 100 microns or 500 microns, before taking the next section, and repeating this process. The result is a series of slides that show sections in “steps” or increments through the tissue.
a. Quick decalcification for H&E staing or histology special staining: Immerse tissue cassette in 11% formic acid with a stir bar overnight in a fume hood, Rinse in running water for 30- 60 minutes (the smell should be gone).
b. Slow EDTA decalcification for Immumostaining: Fixed specimens are rinsed in old EDTA decal solution before placing in Decal Bath. Solution must be stirred continuously. Decal time for mouse bone samples are as follows: Calvaria: 7 days; Arm or legs: 14 days.
Make Solution Recipe (4 Liter): Distilled Water 3 L; Hydroxide, concentrated 280 ml; EDTA (FW 292.2) 400 gm
Dilute 280 ml of concentrated Ammonium Hydroxide in 3L of distilled water. Add 400gm of EDTA and stir till dissolved. Add more concentrated ammonium hydroxide until pH is 7.2. Add more water to make the final volume 4L.

Flow Chat: From 3D Culture Cells to Digital Images
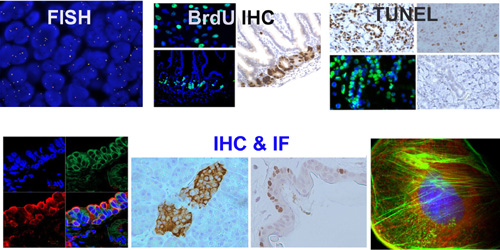
VitroVivo Biotech offers cutting-edge molecular histopathology services, providing comprehensive technical and professional support for biomedical research, pathology, and molecular investigations. Our specialized services enable precise protein, RNA, and DNA analysis within tissue samples, supporting cancer research, neuroscience, developmental biology, and more.
🔬 Immunohistochemistry (IHC) – High-sensitivity protein detection in tissue sections.
🧪 Immunofluorescence (IF) Staining – Single or double antibody staining for cellular and molecular imaging.
🧬 Fluorescence In Situ Hybridization (FISH) – DNA probe-based detection for gene amplification, deletion, or chromosomal abnormalities.
📌 In Situ RNA Hybridization (ISH) – Detection of mRNA, lncRNA, or microRNA, including RNAscope® ISH assays for high-resolution gene expression analysis.
💀 In Situ Apoptosis Detection (TUNEL Assay) – Identification of cell death in tissue samples.
🧩 In Situ BrdU or EdU Cell Proliferation Assay – Visualization of actively dividing cells.
🔄 In Situ Autophagy Detection Assay – Analysis of autophagy-related cellular processes.
✅ State-of-the-Art Techniques – Advanced methodologies for high-quality, reproducible results.
✅ Customizable Solutions – Tailored services to meet specific research and diagnostic needs.
✅ Expert Support – Experienced histopathology specialists ensuring precision and reliability.
📩 Contact VitroVivo Biotech today to discuss your molecular histopathology service needs and receive expert support for your research!
All of the molecular histopathology experiment will include positive control, negative control, and experiment samples. VitroVivo guarantees positive control works well to the intended standard.
In general, we prefer that customers provide primary antibodies for IHC and IF services. VitroVivo will provide other reagents for IHC and IF experiments. If the primary antibodies have been validated by VitroVivo, we are happy to use our antibodies.
Yes, you need to prepare probes. VitroVivo provides other reagents.
You only need to send the samples to us.
Yes, We have customized sample collection services available.
|
|
|
|
|
|
|
|
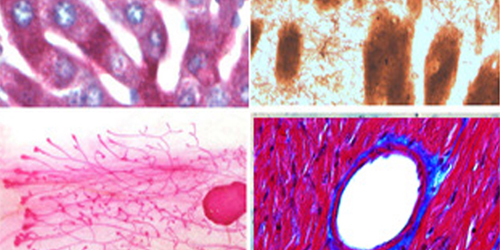
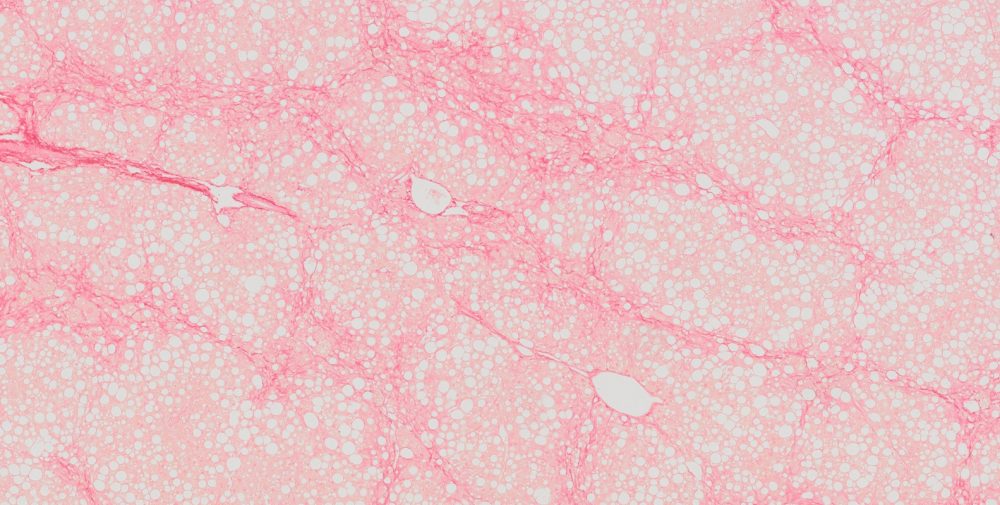
VitroVivo Biotech provides special staining services designed to enhance tissue visualization beyond standard Hematoxylin and Eosin (H&E) staining. Special stains help identify specific tissue structures, cellular components, microorganisms, metals, salts, and more that are not easily detected by routine staining.
🔬 Beyond H&E Staining – Special stains use advanced techniques to differentiate specific tissue components.
🧪 Precision & Customization – Essential for research, pathology, and clinical diagnostics.
🧬 Diverse Applications – Supports studies in histology, microbiology, and molecular biology.
Special stains can be categorized into different groups based on their application:
✔ Histological Stains – Identify structural components within tissue.
✔ Microbial Stains – Detect bacteria, fungi, and other microorganisms.
✔ Histochemical Stains – Highlight specific biomolecules, metals, and deposits.
✔ Advanced Stains – Includes immunohistochemistry (IHC) and in situ hybridization (ISH) for protein and DNA/RNA analysis.
We offer a range of high-quality special stains using standardized protocols:
✅ Mammary Gland Whole Mount Staining
✅ Alcian Blue Hematoxylin/Orange G Staining
✅ Oil Red O Staining (for lipid detection)
✅ Modified Gomori’s Trichrome Staining (for muscle and connective tissues)
✅ Bielschowsky’s Silver Staining (for neural structures)
✅ Masson’s Trichrome Staining (for collagen fibers)
✅ Picro-Sirius Red Staining (for collagen analysis)
✅ Reticulum Staining (for reticular fibers)
✅ Custom Special Stains – Available upon request.
Prefer to perform staining in your own lab? VitroVivo offers special stain kits for self-use, ensuring high-quality and reproducible results.
📩 Contact us at service@vitrovivo.com for inquiries, pricing, or custom staining solutions tailored to your research needs!
Any stain, other than an H&E stain, is classified as a special stain. The common special stains include mammary gland whole mount stain, alcian blue hematoxylin-orange G stain, alcian blue stain, alcian blue – PAS stain, oil red O stain, Nissl stain, Bielschowsky’s silver stain and Masson’s trichrome stain, etc. For more information, please visit our website page of Histochemical Stain Kits and Image Gallery.
This table gives you a guidance of the choice of special staining methods or kits (if you want to perform staining by your self). If you can not find the staining methods from this table, please send your email to service@vitrovivo.com for inquiry, our service team will get back to you as soon as possible.
| Product Name | SKU# | Visualization for | Typical Results |
| Hematoxylin and Eosin Kit | VB-3000 | General morphology of tissue and cell |
|
| Mammary Gland Whole Mount Stain Kit | VB-3001 | Wholemount staining of mouse mammary glands |
|
| Alcian Blue Hematoxylin-Orange G Stain Kit | VB-3002 | Differentiate cartilage, mature bone, and immature bone found in various stages of endochondral ossification and fracture callus |
|
| Alcian Blue Stain Kit | VB-3003 | Tisssue mucosubstances |
|
| PAS Stain Kit | VB-3004 | Glycogen, mucin, and fungi |
|
| Alcian Blue – PAS Stain Kit | VB-3005 | Acidic and neutral mucins as well as mixtures of acidic and neutral mucins |
|
| Luxol Fast Blue Stain Kit | VB-3006 | Myelin including phospholipids and neurons |
|
| Oil Red O Stain Kit | VB-3007 | lipid and fat staining on formalin fixed frozen sections |
|
| Alizarin Red Stain Kit | VB-3008 | Calcium on tissue sections |
|
| Prussian Blue Stain Kit | VB-3009 | Ferric iron on tissue sections |
|
| Nissl Stain Kit | VB-3010 | Neuron Nissl body |
|
| Congo Red Amyloid Stain Kit | VB-3011 | Amyloid deposits |
|
| Sudan Black B Lipid Stain Kit | VB-3012 | Lipid and fat |
|
| Toluidine Blue Stain Kit | VB-3013 | Mast cells |
|
| Modified Gomori’s Trichrome Stain Kit | VB-3014 | Connective fiber |
|
| Bielschowsky’s Silver Stain Kit | VB-3015 | Axons, neurofibrillary tangles and senile plaques |
|
| Masson’s Trichrome Stain Kit | VB-3016 | Collagen and mucus |
|
| Picro-Sirius Red Stain Kit | VB-3017 | Collagen fibers |
|
| Reticulum Stain Kit | VB-3018 | Reticular fibers |
|
| Verhoeff Van Gieson Elastin Stain Kit | VB-3019 | Elastic fibers |
|
| Fontana-Masson Stain Kit | VB-3020 | Melanin pigment and argentaffin granules |
|

FAQ 1. What is cryotomy?
Cryotomy, frozen sectioning or cryo-sectioning is a technique that a cryotome is used to prepare thin and frozen sections for biological tissues. Frozen sections can be used for tissue analysis that allows for rapid interpretation and diagnosis of the tissue during surgery. Cryotomy can also be used in the preparation of sections containing fats and enzymes which can easily be lost in alcohol or paraffin sections.
Flash frozen fresh tissue in OCT is a common method for frozen section preparation. It features:
| Pros | Cons |
|
|
Protocol
Place a drop of Optimal cutting temperature compound (OCT compound) in the bottom of the mold and place the tissue in the OCT. This will hold the tissue in place while you fill the mold with OCT. Just be careful to exclude large bubbles, fill the mold level full, and freeze by one of the methods below.
Use dry ice in pellet form. Place a small stainless steel bowl (or Pyrex or polypropylene beaker) in the bottom of a styrofoam container and fill the space around the bowl with dry ice pellets. Place some pellets in the bowl and slowly add isopentane (2-methyl butane) or acetone. Work in a fume hood, of course, as these are flammable. When the pellets stop bubbling vigorously, the “slurry” is ready. Once you’ve filled the mold and oriented the tissue, immerse it in the liquid to freeze it.
Isopentane also can be chilled in liquid nitrogen (-176ºC). With the liquid nitrogen in a styrofoam container or Dewar flask, use a tongs to lower a stainless steel, Pyrex, or polypropylene container of isopentane into the liquid nitrogen. The isopentane will start to become opaque as it nears freezing. Take the isopentane out of the liquid nitrogen and freeze the specimen as described above. Chill the isopentane again as necessary for subsequent tissues. This method has the advantage of very rapid freezing.
Sometimes we need to fix the tissue first and then do the OCT embedding.
| Pros | Cons |
|
|
Protocol
Step 1
Fixation: Do all steps at 4°C
1. After removal of the tissues from the body, wash briefly in ice cold PBS plus Ca++ and Mg++
2. Fix tissues in fresh (<1wk old) 4% “paraformaldehyde” at 4°C or 10% neutral buffered formalin. The most ideal form of fixation for animal organs involves transcardiac perfusion of PFA prior to removal of the organ from the body.Time of subsequent immersion fixation depends on subsequent steps, but the best morphology is obtained if they are fixed 24 hrs after perfusion or 48-72 hrs if only immersion fixed.
3. Place tissues in 15% sucrose in PBS until tissue sinks (6-12 hrs) and then 30% sucrose in PBS for overnight or until tissue sinks. Best if the tissues are gently nutated, taking care to avoid contact with bubbles and the air surface interface.
Step 2
OCT embedding: can use a slower freeze in crushed powder dry ice alone, or same method as preparation of OCT embedding block for unfixed fresh tissue (see FAQ 2 above).
The frozen blocks can be temporarily stored in dry ice. Transfer the blocks to a liquid nitrogen storage tank (Years) or -80°C freezer (Months).The sample should never be thaw unless there is specific requirement.



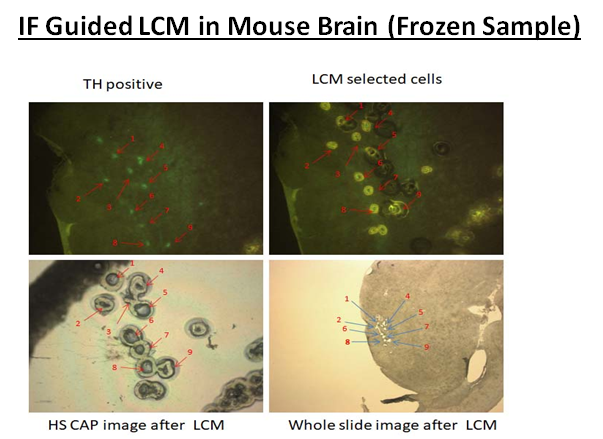
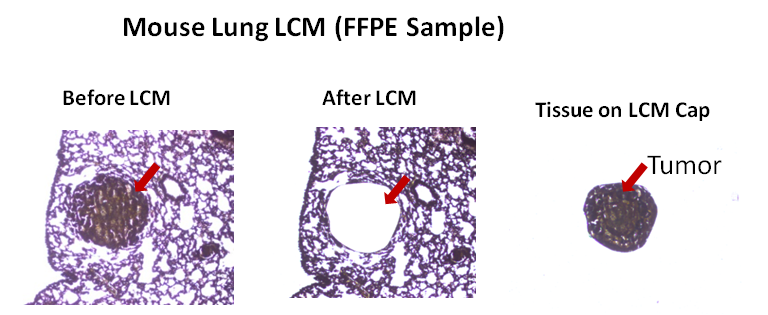
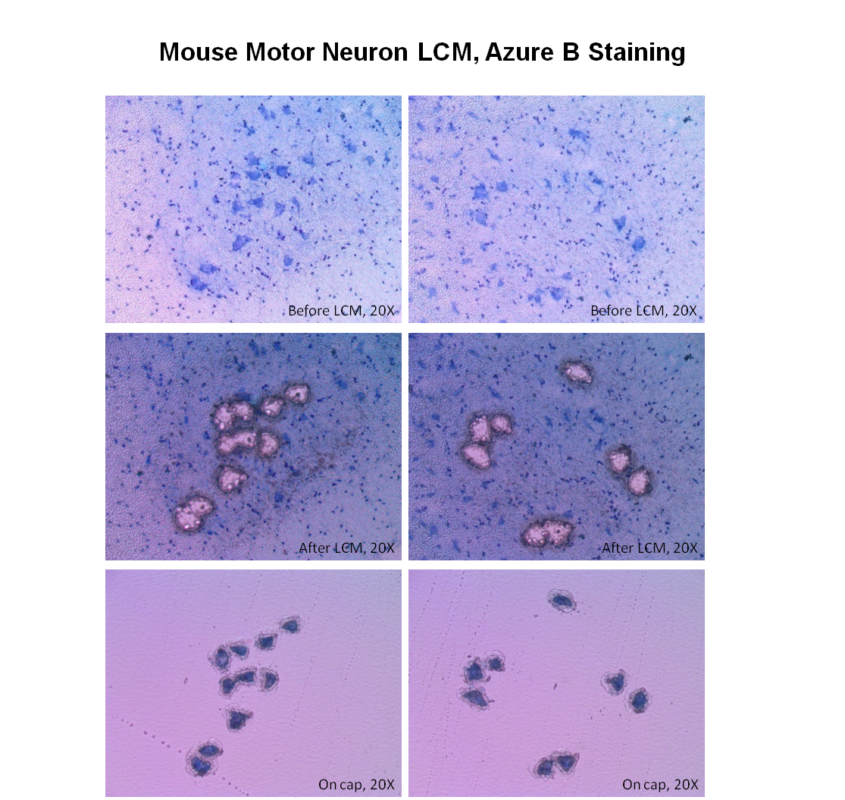
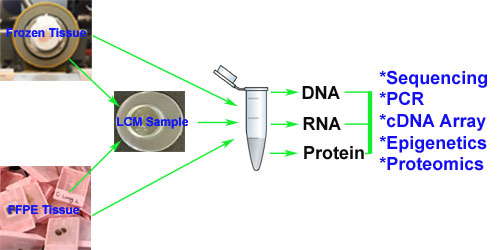
Laser Capture Microdissection (LCM), also known as Laser Microdissection (LMD), is a contact-free and contamination-free technique that enables the precise isolation of specific cells or tissue areas from a wide range of biological samples. This powerful method provides high accuracy and efficiency, making it ideal for genomic, proteomic, and cellular analysis.
VitroVivo Biotech offers advanced Laser Capture Microdissection services to support your research needs. By isolating individual cells or specific regions of tissue, we enable downstream analysis with unmatched precision for applications like next-generation sequencing, PCR, proteomics, and gene expression studies.
✅ Contact-Free and Contamination-Free – Minimizes contamination risk and preserves sample integrity.
✅ High Precision – Laser cutting width of less than 1 µm ensures minimal disruption to surrounding tissue.
✅ Live Cell Viability – Isolate live cells without damaging them, enabling cloning and reculturing.
✅ Preserved Sample Quality – No alteration to the morphology or chemistry of isolated cells, making it ideal for DNA, RNA, and protein analysis.
📩 Contact VitroVivo Biotech today for Laser Capture Microdissection (LCM/LMD) services to elevate your research with high-quality cell isolation and analysis.
LCM or LMD is a method to isolate specific single cells or entire areas of tissue from a wide variety of tissue samples under direct microscopic visualization. LCM or LMD technology can harvest the cells of interest directly or can isolate specific cells by cutting away unwanted cells to generate histologically pure enriched cell populations. A variety of downstream applications exist: DNA genotyping and loss-of-heterozygosity (LOH) analysis, RNA transcript profiling, cDNA library generation, proteomics discovery and signal-pathway profiling.
Yes, please send your request to: service@vitrovuvo.com. You also can visit the page of FFPE, frozen and laser microdissection sample analysis services.
Yes, see the links below: