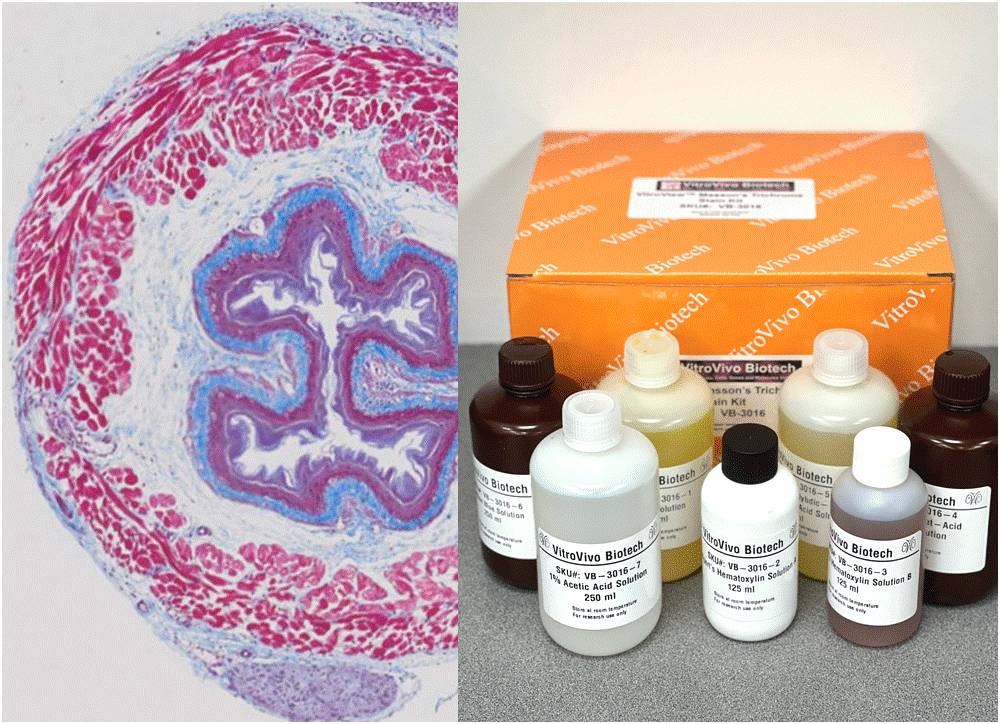
Description
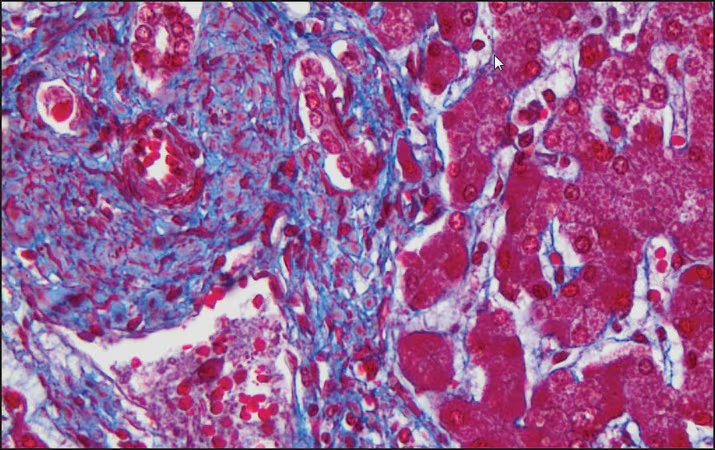
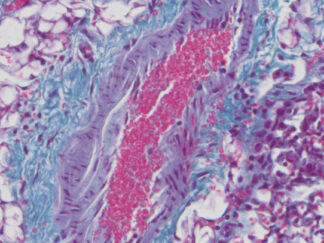
Masson’s Trichrome Stain Kit is used to differentiate between collagen and smooth muscle in tumors, and the increase of collagen in diseases such as cirrhosis. It is a routine stain for liver and kidney biopsies. This kit can be used for FFPE and frozen tissue slides.
Kit Introduction Viedeo
Kit Contents
- VB-3016-1 Bouin’s solution———————————————————250 ml
- VB-3016-2 Weigert’s Hematoxylin Solution A——————————125 ml
- VB-3016-3 Weigert’s Hematoxylin Solution B——————————125 ml
- VB-3016-4 Biebrich Scarlet-Acid Fuchsin Solution———————–250 ml
- VB-3016-5 Phosphomolybdic-Phosphotungstic Acid Solution —250 ml
- VB-3016-6 Aniline Blue Solution————————————————-250 ml
- VB-3016-7 1% Acetic Acid Solution———————————————250 ml
Protocol
For formalin-fixed, paraffin-embedded (FFPE) tissue sections:
- Deparaffinize in Xylene I for 6 minutes and II for 6 minutes.
- Rehydrate: ethanol 100% (2 minutes)x2; ethanol 95% (2 minutes)x2; ethanol 70% (2 minutes)x1; Rinse in distilled water (5 minutes).Mordant in Bouin’s Solution, 60°C for 1 hour.
- Prepare Weigert’s working hematoxylin: mix Weigert’s Hematoxylin Solution A and B at 1:1 ratio.
- Weigert’s working hematoxylin for 10 minutes. (Note: Discard after use.)
- Blue in running tap water for 5 minutes, rinse once for 1 min in distilled water.
- Biebrich Scarlet-Acid Fuchsin Solution for 5 minutes. (Note: Solution may be used twice only, then discarded.)
- Rinse once for 1 min in distilled water.
- Phosphotungstic/phosphomolybdic acid solution for 10 minutes, discard solution.
- Transfer directly into Aniline Blue Solution for 10 minutes.
- Rinse once for 2-3 seconds in distilled water.
- Acetic Acid Solution for 2-3 seconds, discards solution.
- Dehydrate with 2 changes of 95% Ethanol and 2 changes of 100% Ethanol (2 minute per change).
- Clear with 3 changes of xylene (5 minutes per change) and coverslip with Permount or other suitable organic mounting medium.
For Frozen Sections:
- Fix frozen sections in 10% formalin (not Zinc) for 30 minutes.
- Rinses in distilled water 3×3min.
- Mordant in Bouin’s Solution, 60°C for 1 hour.
- Wash in running tap water for 5 minutes to remove the picric acid.
- Weigert’s working hematoxylin for 7 minutes. (Note: Discard after use.)
- Blue in running tap water for 5 minutes, rinse in distilled water.
- Biebrich Scarlet-Acid Fuchsin Solution for 5 minutes. (Note: Solution may be used twice only, then discarded.)
- Rinse in distilled water.
- Phosphotungstic/phosphomolybdic Acid Solution for 10 minutes, discard solution.
- Transfer directly into Aniline Blue Solution for 5 minutes.
- Rinse once for 2-3 seconds in distilled water.
- 1% Acetic acid for 2-3 seconds , discard solution.
- Dehydrate with 2 dip of 95% Ethanol and 2 changes of 100% Ethanol (2 minutes per change).
- Clear with 3 changes of xylene (5 minutes per change) and coverslip with Permount or other suitable organic mounting medium.
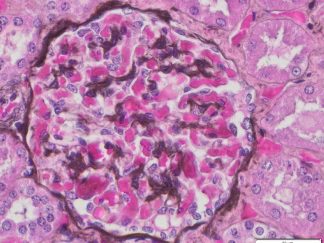
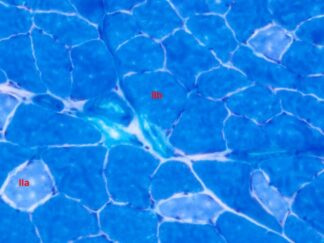
Expected results
- Cytoplasm, keratin, muscle fibers, Erythrocytes ——— red
- Nuclei ———————————————————————— black
- Collagen and mucus ————————————————- blue
More Images
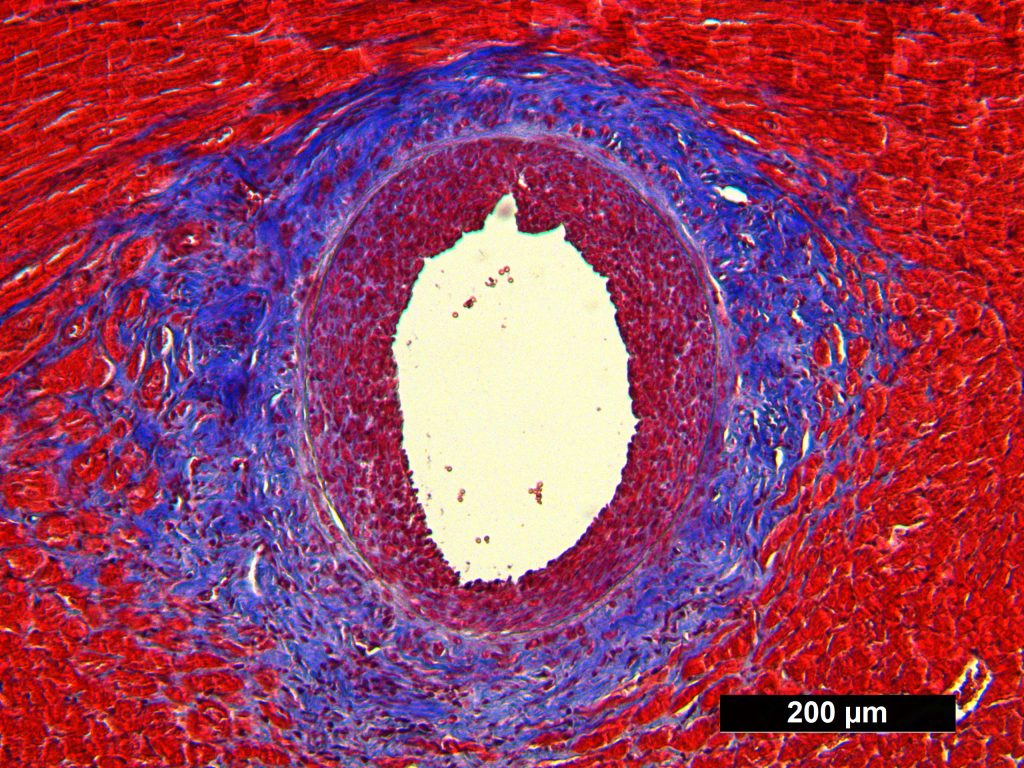
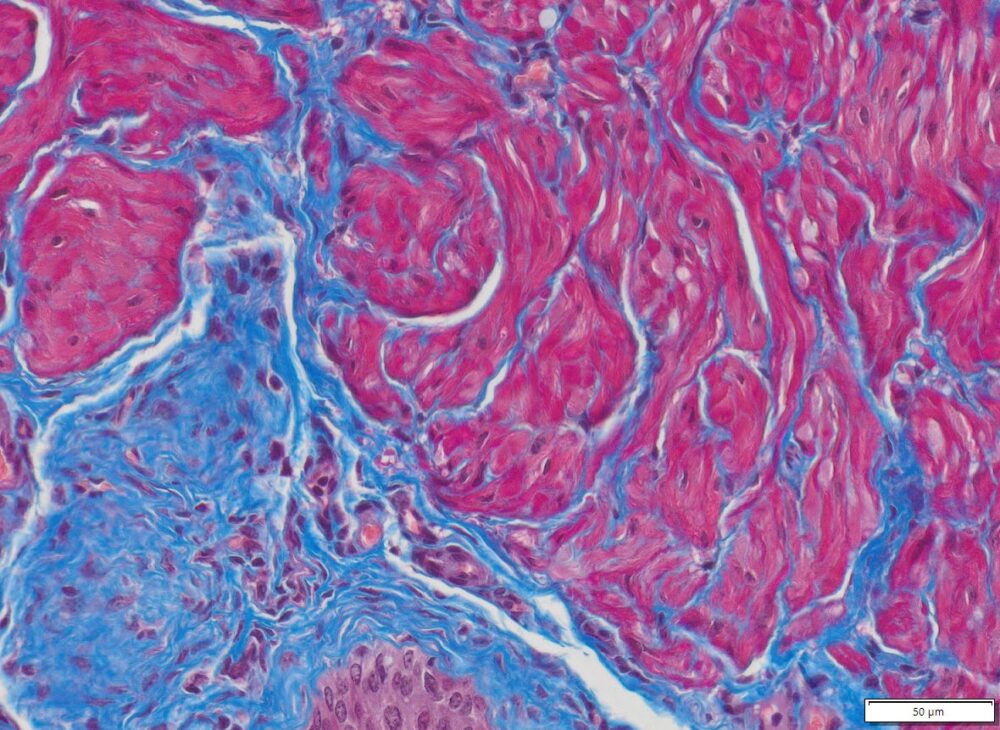
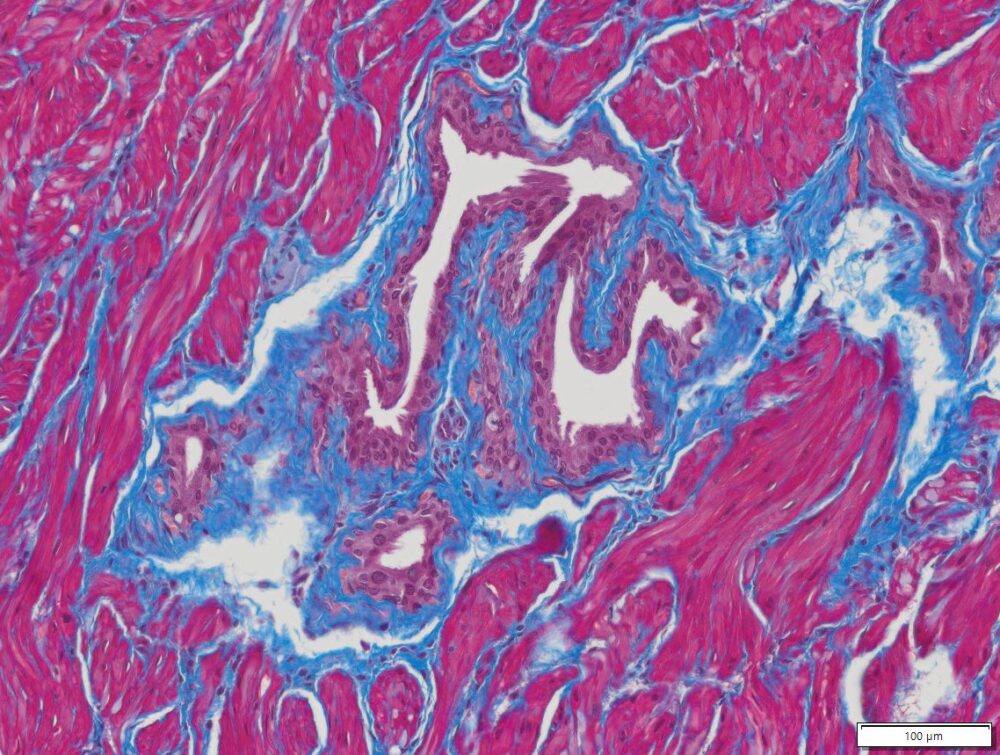
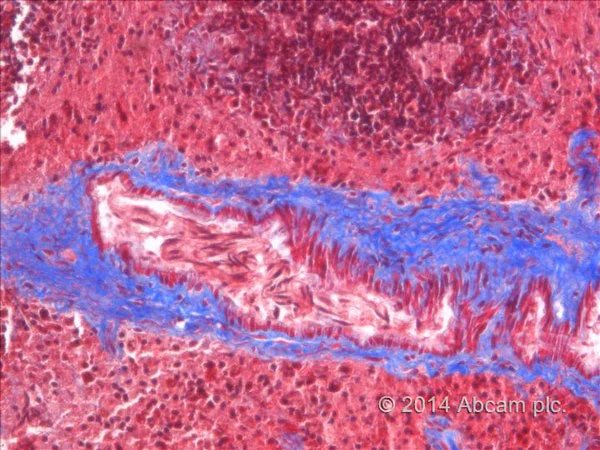
Masson Trichrome Staining, Rat Heart |
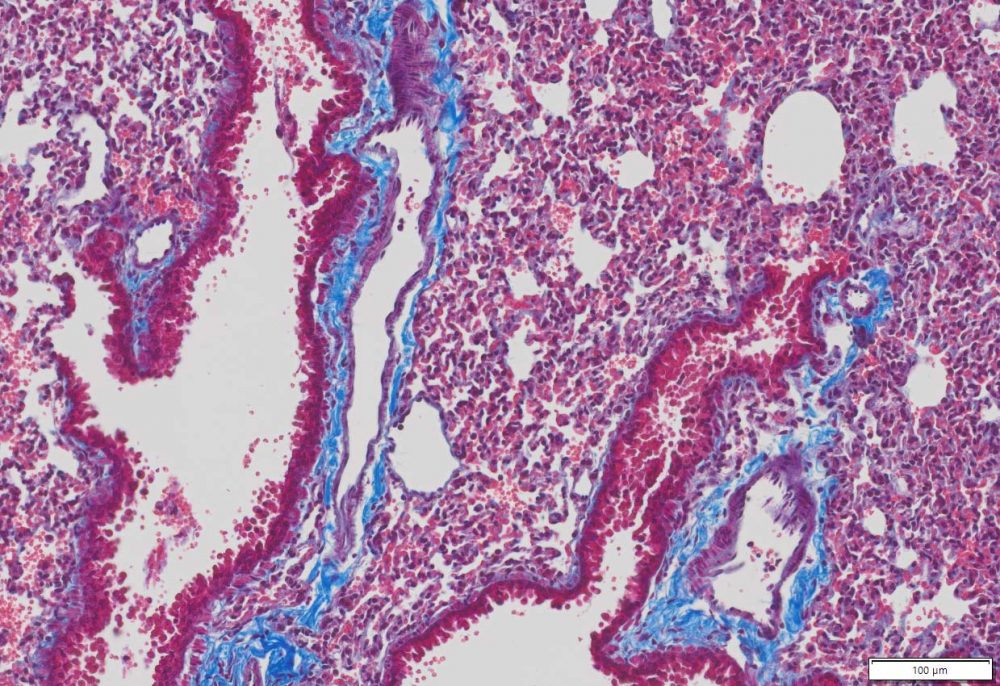
Masson Trichrome Staining, Mouse Lung |
|
|
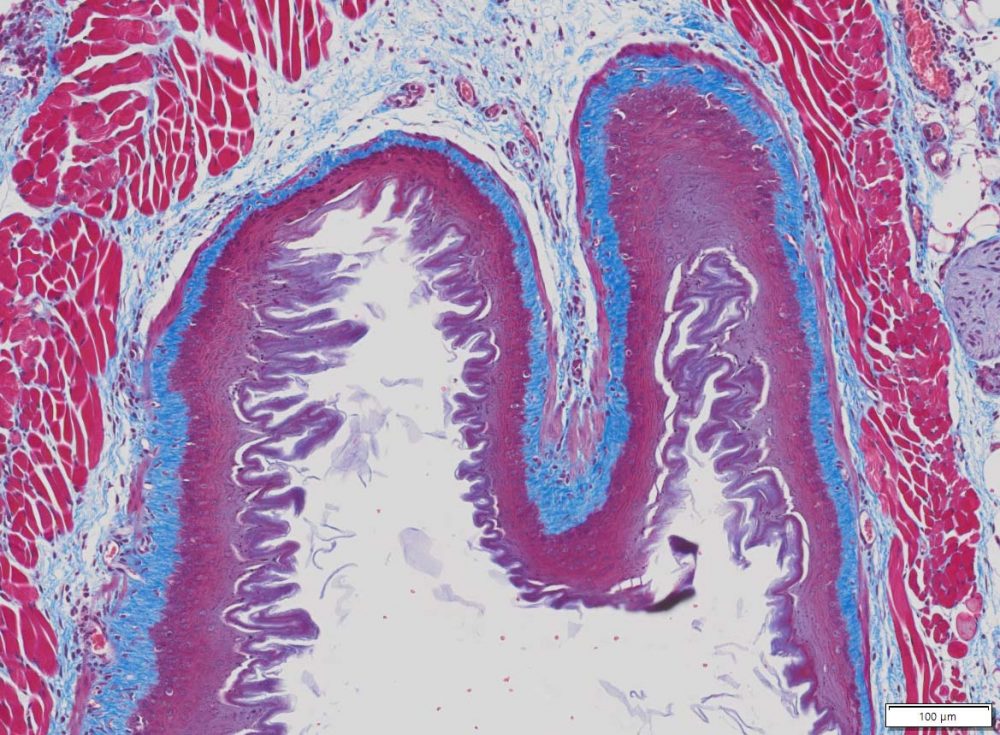
Masson Trichrome Staining, Mouse Esophagus |
Masson’s Trichrome Stain Kit Comparison Among Different Vendors
| Vendor / Catalog# | VitroVivo Biotech / VB-3016 | Abcam / ab150686 | Millipore-Sigma / HT15 |
| Components | 1. Bouin’s Solution (1 x 250 mL) 2. Weigert’s Hematoxylin Solution A (1 x 125 mL) 3. Weigert’s Hematoxylin Solution B (1 x 125 mL) 4. Biebrich Scarlet-Acid Fuchsin Solution (1 x 250 mL) 5. Phosphomolybdic-Phosphotungstic Acid Solution (1 x 250 mL) 6. Aniline Blue Solution (1 x 250 mL) 7. 1% Acetic Acid Solution (1 x 250 mL) |
1. 1% Acetic Acid Solution (1 x 125 mL) 2. Aniline Blue Solution (1 x 125 mL) 3. Biebrich Scarlet/Acid Fuchsin Solution (1 x 125 mL) 4. Bouin’s Fluid (1 x 125 mL) 5. Phosphomolybdic/Phosphotungstic Acid Solution (1 x 125 mL) 6. Weigert’s Iron Hematoxylin A (1 x 125 mL) 7. Weigert’s Iron Hematoxylin B (1 x 125 mL) |
1. Biebrich Scarlet-Acid Fuchsin Solution (Cat. No. HT151-250ML) 2. Phosphotungstic Acid Solution (Cat. No. HT152-250ML) 3. Phosphomolybdic Acid Solution (Cat. No. HT153-250ML) 4. Aniline Blue Solution (Cat. No. HT154-250ML) |
| Website | https://vitrovivo.com/product/massons-trichrome-stain-kit/ | https://www.abcam.com/en-us/products/assay-kits/trichrome-stain-kit-connective-tissue-stain-ab150686 | https://www.sigmaaldrich.com/US/en/product/sigma/ht15#product-documentation |
| List Price (USD) | $369 / kit | $505 / kit | $429 / kit |
| Tests per Kit (1 mL/slide) | 250 slides | 125 slides | 250 slides |
| Cost per Slide (1 mL/slide) | $1.48 / slide | $4.04 / slide | $1.72 / slide, plus cost of other reagents |
| Quality/Staining Images |
|
|
|
Peer-reviewed publications and preprints that have cited the use of the VitroView™ Masson’s Trichrome Stain Kit (VB‑3016) kit
-
Ko KW, et al. (2021) — Integrated bioactive scaffold with polydeoxyribonucleotide and stem-cell-derived extracellular vesicles for kidney regeneration.
ACS Biomaterials Science & Engineering 15(4):7575–7585.
Uses VB-3016 Masson’s Trichrome to assess collagen and connective tissue in kidney tissue. VitroVivo -
Park SY, et al. (2024) — Multifunctional vitamin D-incorporated PLGA scaffold with BMP/VEGF-overexpressed tonsil-derived MSC via CRISPR/Cas9 for bone tissue regeneration.
Materials Today Bio 28:101254.
Trichrome staining with VB-3016 was employed to evaluate collagen deposition during bone regeneration. VitroVivo -
Lee JK, et al. (2024) — Osteoporotic bone regeneration via plenished biomimetic PLGA scaffold with sequential release system.
Small 20:2310734.
VB-3016 was used to visualize connective tissue and collagen in remodeled bone sections. VitroVivo -
Barkovskaya A, et al. (2025) — Inhibition of PIKfyve kinase induces senescent cell death…, mouse model of idiopathic pulmonary fibrosis.
bioRxiv preprint (2025.03.19.644224).
Masson’s Trichrome with VB-3016 was applied to examine fibrosis and collagen remodeling in lung tissue. VitroVivo -
Nam SH, et al. (2024) — Glycinamide facilitates nanocomplex formation… promotes osteoblast differentiation in vivo.
Biomaterials Advances 10:947-959.
Collagen deposition in bone and connective tissues was evaluated with the VitroView trichrome kit. VitroVivo -
Bui HTD, et al. (2023) — Korean amberjack skin-inspired hyaluronic acid bioink for reconstruction of human skin.
ACS Omega 8(25):22752-22761.
VB-3016 was used to highlight collagen and connective tissue in engineered skin constructs. VitroVivo -
Kim DS, et al. (2022) — Controlled vitamin D delivery with injectable hyaluronic acid-based hydrogel for restoration of tendinopathy.
Journal of Tissue Engineering 13:1177/20417314221122089.
Trichrome was used to assess collagen fiber organization in tendon repair. VitroVivo -
Kim DS, et al. (2021) — Advanced PLGA hybrid scaffold with a bioactive PDRN/BMP2 nanocomplex for angiogenesis and bone regeneration…
Science Advances 7:eabj1083.
Collagen deposition and tissue integration were evaluated with VB-3016 trichrome staining. VitroVivo -
Baek SW, et al. (2023) — Continuous NO dual-generation by ZnO nanoparticle conjugated with α-lipoic acid for functional biodegradable vascular stent.
Chemical Engineering Journal 470:144174.
Masson’s Trichrome was used for connective tissue and collagen analysis in vascular tissues. VitroVivo -
Lee S, et al. (2024) — Injectable microparticle-containing hydrogel with controlled release of bioactive molecules for facial rejuvenation.
Materials Today Bio 24:100890.
Collagen and extracellular matrix remodeling were visualized using VB-3016. VitroVivo -
Meca-Laguna G, et al. (2025) — γδ T Cells Target and Ablate Senescent Cells in Aging and Alleviate Pulmonary Fibrosis.
bioRxiv preprint (2025.05.05.652251).
Trichrome staining with VB-3016 was used to evaluate collagen deposition in lung fibrosis. VitroVivo -
Hypertension-induced heart failure disrupts cardiac sympathetic innervation — PMC Article.
This paper reports using the VitroView Masson’s Trichrome Stain Kit (VB-3016) to perform trichrome staining on cryosections of cardiac tissue. PMC
Why Choose the VitroView™ Masson’s Trichrome Stain Kit for Your Research?
- Cost-effective
- Easy to use
- High quality with strict quality control and assurance
- Validated and trusted by numerous peer-reviewed publications
Note: This Trichrome Stain Kit is intended for research purposes only. This product is not intended to be used for therapeutic or diagnostic purposes in humans or animals.
Precautions: Handle with care. Avoid contact with eyes, skin and clothing. Do not ingest. Wear gloves.