Welcome to VitroVivo Biotech
About VitroVivo Biotech
VitroVivo Biotech is a leading innovator in the development and commercialization of advanced histological products and services. Founded in 2012 by a team of expert biomedical scientists, we are located in the I-270 corridor of Montgomery County, Maryland.
Our mission is to provide cutting-edge expertise in histology and molecular histopathology, accelerating scientific discovery for biomedical research institutions, biotech companies, and pharmaceutical organizations. At VitroVivo, we specialize in making tissues, cells, genes, and molecules visible, advancing the understanding of biological systems.
Why Choose VitroVivo Biotech Services and Products?
-
All-in-One Histology & Molecular Pathology Services
VitroVivo offers a full suite of high-quality services, including Laser Capture Microdissection (LCM), immunostaining, FISH/ISH, and expert analysis by certified pathologists—delivering precise, reliable results for advanced biomedical research. -
High-Quality Kits & Reagents
We supply a broad range of trusted histology and molecular pathology kits, with global distribution across the U.S., Europe, and Asia-Pacific. -
Trusted by Top Institutions
Our clients include NIH labs, leading universities, and major biotech and pharmaceutical companies—reflecting our reputation for excellence. -
Proven Scientific Impact
VitroVivo’s services and products are cited in numerous peer-reviewed publications, highlighting our role in advancing research.
At VitroVivo Biotech, we’re dedicated to supporting scientific discovery and improving human health through innovative tools and expert solutions.
Products
-
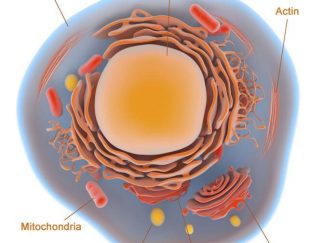
Cell and Organelle Stains
-
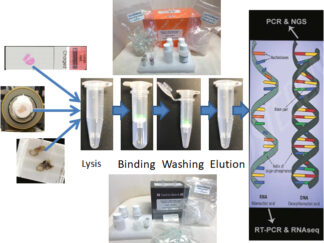
DNA/RNA Isolation Kits for FFPE and Frozen or Fresh Samples
-

FFPE Blocks and Sections
-
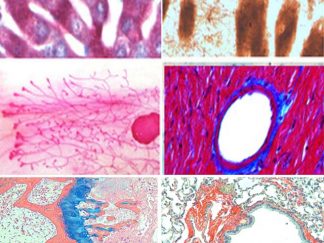
Histochemical Stains
-

Histology Consumables
-

Histology Supplies
-
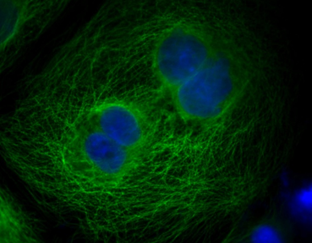
Immunofluorescence Detection Kits
-
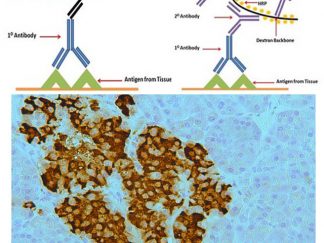
Immunohistochemical Detection Kits
-
In Situ Detection of Cell Biology
-
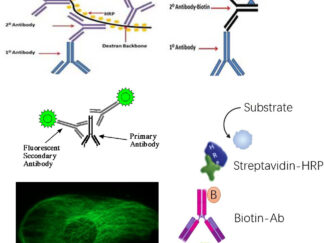
Polymer HRP-Conjugated, Biotinylated, and Fluorescently Labeled Secondary Antibodies, Streptavidin, and Nucleotides
-
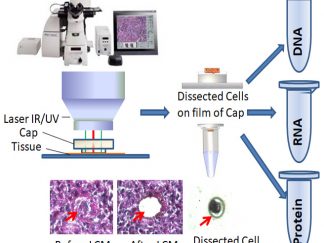
Pure Cell Population Isolated through LCM
-
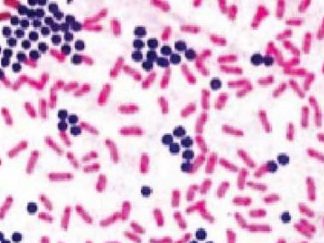
Special Stain Kit for Pathogen Identification
-
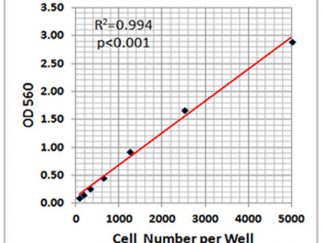
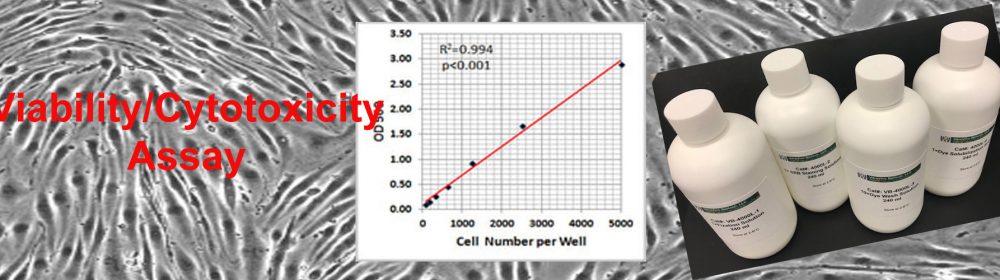
SRB Viability/Cytotoxicity Assay Kit
Services
|
 Histopathology and Laser Capture Microdissection Services Histopathology and Laser Capture Microdissection Services |
Video of VitroVivo Biotech’s Product and Service Overview (YouTube)
 |
 |
Our Lab Facility
 |
 |
 |
 |
Selected Clients from USA
(Click on the logo to be linked to the institution or company)
Government Agencies
Universities/Academic Research Organizations
Pharmaceutical and Biotechnology Companies
International Clients from
 Canada Canada |
 P.R. China P.R. China |
 Japan Japan |
 South Korea South Korea |
 Taiwan Taiwan |
 Hongkong Hongkong |
 India India |
 Vietnam Vietnam |
 German German |
 United Kingdom United Kingdom |
 Switzerland Switzerland |
 Italy Italy |
 Latvia Latvia |
 Austria Austria |
 France France |
Selected Publications Citing VitroVivo’s Services and Products from the Recent Literature
- Barbosa MMP, et al. (2024), Anchored immunotherapy with alum-tethered canine interleukin-12 (JEN-101) is safe and induces therapeutic responses: results of a preclinical study. JITC, 1316.
- Wako M, et al. (2024), “Sulfated polysaccharides isolated from nacre extract suppress chronic scopolamine administration-induced amyloid-beta deposition,” Applied Sciences. 14(17), 7830
- Shim KS, et al. (2024), Rosmarinic Acid Ameliorates Dermatophagoides farinae Extract-Induced Atopic Dermatitis-like Skin Inflammation by Activating the Nrf2/HO-1 Signaling. Int. J. Mol. Sci. 2024, 25(23), 12737;
- Najera SS, et al. (2024), Targeting NAD+ Metabolism Vulnerability in FH-Deficient Hereditary Leiomyomatosis and Renal Cell Carcinoma with the Novel NAMPT Inhibitor OT-82. Molecular Cancer. DOI: 10.1158/1535-7163.MCT-24-0225
- Pan T, et al. (2024), BIGH3 mediates apoptosis and gap junction failure in osteocytes during renal cell carcinoma bone metastasis progression. Cancer Letters. 596, 217009
- Kang M, et al. (2024), Dietary supplementation with Lacticaseibacillus rhamnosus IDCC3201 alleviates sarcopenia by modulating the gut microbiota and metabolites in dexamethasone-treated models. Food & Function. 15, 4936-4953
- Shirazi M, et al. (2024), Altered kidney distribution and loss of ACE2 into the urine in acute kidney injury. American Journal of Physiology. 327 (3), F412-F425
- Kanemaru E, et al. (2024), Exclusion of sulfide: quinone oxidoreductase from mitochondria causes Leigh-like disease in mice by impairing sulfide metabolism. The Journal of Clinical Investigation. ;134(15):e170994
- Mahmoudi Aliabadi P, et al. (2024), Periodic Acid Schiff Staining to Detect Mott Cells, Aberrant Plasma Cells Containing Immunoglobulin Inclusions Called Russell Bodies. Current Pathology.4, e70005
- Park H, et al. (2024), Evaluation of liver function using Cordyceps militaris extract powder in Sprague-Dawley rat with acute hepatic injury induced by dimethylnitrosamine. Korean Journal of Physiology. 51(2),147-158
- Ogunlusi O, et al. (2024), Disruption of Circadian Clock Induces Abnormal Mammary Morphology and Aggressive Basal Tumorigenesis by Enhancing LILRB4 Signaling. bioRxiv. doi: https://doi.org/10.1101/2024.03.19.585534
- Kumar K, et al. (2024), Simulated Galactic Cosmic Radiation Exposure-Induced Mammary Tumorigenesis in ApcMin/+ Mice Coincides with Activation of ERα-ERRα-SPP1 Signaling. Cancers. 16(23), 3954
- Shrestha UM, et al. (2024), A Feasibility Study of [18F]F-AraG Positron Emission Tomography (PET) for Cardiac Imaging–Myocardial Viability in Ischemia–Reperfusion Injury Model. Molecular Imaging and Biology. 26,869–878
- Huang S, et al. (2024), Comparative Responses to Demethylating Therapy in Animal Models of Osteosarcoma. Research Square. https://doi.org/10.21203/rs.3.rs-4451060/v1
- Scalco A, et al. (2024), Hypertension-Induced Heart Failure Disrupts Cardiac Sympathetic Innervation. American Journal of Physiology. 327(6): H1544-H1558
- Chae HD, et al. (2024). A Feasibility Study of [18F] F-AraG Positron Emission Tomography (PET) for Cardiac Imaging–Myocardial Viability in Ischemia-Reperfusion Injury Model. Research Square. doi: 10.21203/rs.3.rs-4244476/v1
- Omachi T, et al. (2024), Effect of Nacre Extract from Pearl Oyster Shells Against Behavioral and Psychological Symptoms of Dementia in Senescence-Accelerated Mouse P8 (SAMP8). Journal of Functional Foods. 116, 106208
- Choe MS, et al. (2024). Simple Modeling of Familial Alzheimer’s Disease Using Human Pluripotent Stem Cell-Derived Cerebral Organoid Technology. Stem Cell Research & Therapy. 15, 118
- Fabian KP, Santiago-Sanchez G, et al. (2024). Alum-Anchored IL-12 Combined with Cytotoxic Chemotherapy and Immune Checkpoint Blockade Enhanced Antitumor Immune Responses in Head and Neck Cancer. Journal for ImmunoTherapy of Cancer. 12(10):e009712
- Wu Y, et al. (2024). Elucidating the Mechanism of Traditional Chinese Medicine Formula (Yifei-Sanjie Pill) in Alleviating the Chemobrain Based on Network Pharmacology. Journal of Traditional Medicine. , https://doi.org/10.1016/j.jtcme.2024.11.008
- Nam SH, et al. (2024). Glycinamide Facilitates Nanocomplex Formation and Functions Synergistically with Bone Morphogenetic Protein 2 to Promote Osteoblast Differentiation In Vitro. Tissue Engineering and Regenerative Medicine. 21, 1093–1107
- Sassu E, et al. (2024). Age-Related Structural and Functional Changes of the Intracardiac Nervous System. Journal of Molecular and Cellular Cardiology. 187,1-14
- Gorbenko N, et al. (2024). Perfusion Bioreactor Conditioning of Small-Diameter Plant-Based Vascular Grafts. Tissue Engineering and Regenerative Medicine.21,1189-1201
- Wasi M, et al. (2024), Mitigating Aging and Doxorubicin-Induced Bone Loss in Mature Mice via Mechanobiology-Based Treatments. Bone. 188, 117235
- Park SY, et al. (2024), Multifunctional Vitamin D-Incorporated PLGA Scaffold with BMP/VEGF-Overexpressed Tonsil-Derived MSC via CRISPR/Cas9 for Bone Tissue Regeneration. Materials Today Bio.28,101254
- Zabransky DJ, et al. (2024), Fibroblasts in the Aged Pancreas Drive Pancreatic Cancer Progression. Cancer Research. 84 (8): 1221–1236.
- Shin S, et al. (2024), Anti-Inflammatory Effects of Aptamin C in Pulmonary Fibrosis Induced by Bleomycin. Pharmaceuticals.17(12),1577
- Fitzsimmons CM, et al. (2024), “Rewiring of RNA Methylation by the Oncometabolite Fumarate in Renal Cell Carcinoma,” NAR Cancer. 6, zcae004
- Batool M, et al. (2024), Early Versus Late Caffeine and/or Non-Steroidal Anti-Inflammatory Drugs (NSAIDS) for Prevention of Intermittent Hypoxia-Induced Neuroinflammation in the Neonatal Rat. International Journal of Molecular Sciences.84(3), 227-250
- Yamamoto H, et al. (2024), Oral Administration of Nacre Extract from Pearl Oyster Shells Has Anti-Aging Effects on Skin and Muscle, and Extends the Lifespan in SAMP8 Mice. Pharmaceuticals. 17(6),713
- Lee JK, et al. (2024), Osteoporotic Bone Regeneration via Plenished Biomimetic PLGA Scaffold with Sequential Release System. Nano Micro Small. 20(3),2310734
- Kim Y, et al. (2024), Discovery and Optimization of a Series of Vinyl Sulfoximine-Based Analogues as Potent Nrf2 Activators for the Treatment of Multiple Sclerosis. Journal of Medicinal Chemistry.46(19),17866-17892
- Kim M, et al. (2024), Early Involvement of Peripherally Derived Monocytes in Inflammation in an NMO-like Mouse Model. Scientific Reports.14, 1177
- Yang X, et al. (2024), Aldehydes Alter TGF-β Signaling and Induce Obesity and Cancer. Cell Reports.43 (9), 114676
- Passos Barbosa MM, et al. (2024), Preclinical Evaluation of an Anchored Immunotherapy Strategy with Aluminum Hydroxide–Tethered IL-12 in Dogs with Advanced Malignant Melanoma. Molecular Cancer Research. https://doi.org/10.1158/1535-7163.MCT-24-0317
- Lee S, et al. (2024), Injectable Microparticle-Containing Hydrogel with Controlled Release of Bioactive Molecules for Facial Rejuvenation. Materials Today Bio.24,100890
- Good CJ, et al. (2024), Uncovering Lipid Dynamics in Staphylococcus aureus Osteomyelitis Using Multimodal Imaging Mass Spectrometry. Cell Chemical Biology. 31 (10),P1852-1868 E5
- Wang S, et al. (2024), Physical Intervention and Data-Driven Biomechanics in Breast Cancer Bone Metastases. ProQuest Dissertations & Theses. https://www.proquest.com/openview/0e40df4b6c2b0bc6b436cdee636366af/1?pq-origsite=gscholar&cbl=18750&diss=y
- Li Y, et al (2023). A novel recessive mutation in OXR1 is identified in patient with hearing loss recapitulated by the knockdown zebrafish. Human Molecular Genetics, 32(5): 764-772.
- Pandey M, et al (2023). Specific regulation of mechanical nociception by Gβ5 involves GABA-B receptors. JCI insight, 8(13): e134685.
- Gunawardhana KL, et al (2023). A systems biology approach identifies the role of dysregulated PRDM6 in the development of hypertension. The Journal of Clinical Investigation,133(4): e160036.
- Dang X, et al (2023). Activating mitofusins interrupts mitochondrial degeneration and delays disease progression in SOD1 mutant amyotrophic lateral sclerosis. Human molecular genetics, 32(7): 1208-1222.
- Lee JK, et al (2023). Nitric Oxide-Releasing Bioinspired Scaffold for Exquisite Regeneration of Osteoporotic Bone via Regulation of Homeostasis. Advanced Science,10(6): 2205336.
- Schmidt LS, et al (2023). PRDM10 RCC: A Birt-Hogg-Dubé-like Syndrome Associated with Lipoma and a Highly Penetrant, Aggressive Renal Tumors Morphologically Resembling Type 2 Papillary Renal Cell Carcinoma. Urology, 179: 58-70.
- Bui HTD, et al (2023). Korean Amberjack Skin-Inspired Hyaluronic Acid Bioink for Reconstruction of Human Skin. ACS Omega. 8 (25): 22752-22761.
- Fitzsimmons CM, et al (2023). Rewiring of RNA methylation by the oncometabolite fumarate in renal cell carcinoma. bioRxiv, 2023-04(doi: https://doi.org/10.1101/2023.04.10.536262)
- Yamamoto H, et al (2023). Nacre extract from pearl oyster shell prevents D-galactose-induced brain and skin aging. Marine Biotechnology, 25(4): 503-518.
- Giri S, et al (2023). COP9 Signalosome Promotes Neointimal Hyperplasia via Deneddylation and CSN5-Mediated Nuclear Export. bioRxiv, 2023-04.(https://doi.org/10.1101/2023.04.11.536468)
- Baek SW, et al (2023). Continuous NO Dual-Generation by ZnO nanoparticle conjugated with α-Lipoic acid for functional biodegradable vascular stent. Chemical Engineering Journal, 470: 144174.
- Carrillo P, et al (2023). The synthetic molecule stauprimide impairs cell growth and migration in triple-negative breast cancer. Biomedicine & Pharmacotherapy. 158:114070.
- Wang J, et al (2023). Circ_0042986 Presence Restrains Cervical Cancer Development via Upregulating PEG3 by Directly Targeting miR-582-3p. Reprod. Sci. 30: 890–902.
- Kumar K, et al (2023). Simulated galactic cosmic radiation (GCR)-induced expression of Spp1 coincide with mammary ductal cell proliferation and preneoplastic changes in ApcMin/+ mouse. Life Sciences in Space Research, 36: 116-122.
- Batool M, et al (2023). Early Versus Late Caffeine and/or Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) for Prevention of Intermittent Hypoxia Induced Neuroinflammation in the Neonatal Rat. Preprints 2023,2023090432[https://doi.org/10.20944/preprints202309.0432.v1].
- Choe MS, et al (2023). Simple modeling of Alzheimer’s disease using human pluripotent stem cell-derived cerebral organoid technology. Research Square [https://doi.org/10.21203/rs.3.rs-2817666/v1].
- Crooks DR, et al (2023). Cryptic splice mutation in the fumarate hydratase gene in patients with clinical manifestations of Hereditary Leiomyomatosis and Renal Cell Cancer. Human Molecular Genetics, 32(22): 3135-3145.
- Franks SE, et al (2023). Exploiting docetaxel-induced tumor cell necrosis with tumor targeted delivery of IL-12. Cancer Immunology, Immunotherapy, 72: 2783–2797.
- Song HK, et al (2023). Extract Ameliorates Atopic Dermatitis–Like Symptoms by Suppressing Proinflammatory Chemokine Production In Vivo and In. Inflammatory immune disease: Molecular mechanisms, translational approaches and therapeutics volume II, 13: 919230.
- Gorbenko Nicole, et al (2023). Small-Caliber Vascular Grafts Engineered from Decellularized Leaves and Cross-Linked Gelatin. Tissue Engineering Part A. 29(13-14):397-409.
- Roh EJ, et al (2023). Multimodal therapy strategy based on a bioactive hydrogel for repair of spinal cord injury. Biomaterials, 299: 122160.
- Amara, et al (2023). Assessment of lipid and pigment content in suspicious melanocytic lesions to improve melanoma detection. Melanoma Research 33(4): 283-292.
- Omachi T, et al (2023). Nacre extract from pearl oyster suppresses LPS-induced depression and anxiety. Journal of Functional Foods. 100: 105373.
- Feng X, et al (2023). Granulocytic myeloid-derived suppressor cells to prevent and treat murine immune-mediated bone marrow failure. Blood Adv. 7(1): 73-86.
- Kanemaru E, et al. (2023). Intranasal administration of polysulfide prevents neurodegeneration in spinal cord and rescues mice from delayed paraplegia after spinal cord ischemia. Redox Biology. 60: 10262.
- Gunawardhana K, et al (2023). A systems biology approach identifies the role of dysregulated PRDM6 in the development of hypertension. J Clin Invest. 133(4): e160036.
- Ko Y, et al (2023). Nonanimal Euglena gracilis-Derived Extracellular Vesicles Enhance Skin-Regenerative Wound Healing. Adv Mater Interfaces. 10: 2202255.
- Kim H, et al(2023). Inhibiting 5-lipoxygenase prevents skeletal muscle atrophy by targeting organogenesis signaling and insulin-like growth factor-1. 13 (6): 3062-3077.
- Kwon OW, et al (2023). Korean Red Ginseng and Rb1 facilitate remyelination after cuprizone diet-induced demyelination. Journal of Ginseng Research. 47(2): 319-328.
- Groarke EM,et al (2023). Efficacy of JAK1/2 inhibition in murine immune bone marrow failure. Blood 141 (1): 72–89.
- Weon S, et al (2023). Extracellular PPM1A promotes mineralization of osteoblasts differentiation in ankylosing spondylitis via the FOXO1A-RUNX2 pathway. Journal of Cellular and Molecular Medicine , 27 (5): 650-658
- Wong V, et al (2023). The Development of Small-Caliber Vascular Grafts Using Human Umbilical Artery: An Evaluation of Methods. Tissue Engineering Part C: Methods. 29 (1):1-10.
- Dang X, et al (2023). Activating mitofusins interrupts mitochondrial degeneration and delays disease progression in SOD1 mutant amyotrophic lateral sclerosis. Human Molecular Genetics, 20;32(7):1208-1222
- Piao C, et al (2022). Pulmonary delivery of a recombinant RAGE antagonist peptide derived from high-mobility group box-1 in a bleomycin-induced pulmonary fibrosis animal model. Journal of Drug Targeting. 30 (7): 792-799
- Kim, et al( 2022) . Terminalia chebula Retz. extract ameliorates the symptoms of atopic dermatitis by regulating anti-inflammatory factors in vivo and suppressing STAT1/3 and NF-ĸB signaling in vitro. Phytomedicine.104:154318
- Kumar K, et al (2022). Simulated galactic cosmic radiation (GCR)-induced expression of Spp1 coincide with mammary ductal cell proliferation and preneoplastic changes in ApcMin/+ mouse. Life Sciences in Space Research. 36: 116-122.
- Yotsuya Y, et al (2022). Nacre extract from pearl oyster attenuates amyloid beta-induced memory impairment. Journal of Natural Medicines . 76: 419–434.
- Heo Y, et al (2022). Bioactive PCL microspheres with enhanced biocompatibility and collagen production for functional hyaluronic acid dermal fillers. Biomaterials. Sci., 10: 947-959.
- Park SJ, et al (2022). In vivo Preclinical Tumor-Specific Imaging of Superparamagnetic Iron Oxide Nanoparticles Using Magnetic Particle Imaging for Cancer Diagnosis. International Journal of Nanomedicine. 17: 3711-3722.
- Maio N, et al (2022). TEMPOL inhibits SARS-CoV-2 replication and development of lung disease in the Syrian hamster model. iScience, 25(10): 105074.
- Shakya M, et al (2022). The G209R Mutant Mouse as a Model for Human PCSK1 Polyendocrinopathy. Endocrinology, 163(5: ,bqac024.
- Song HK, et al (2022). Spatholobus suberectus Dunn Water Extract Ameliorates Atopic Dermatitis–Like Symptoms by Suppressing Proinflammatory Chemokine Production In Vivo and In Vitro. Front Pharmacol. 13: 919230.
- Antony R, et al (2022). UCHL1 Regulates Lipid and Perilipin 2 Level in Skeletal Muscle. Front. Physiol, 13: 855193.
- Bhatia R, et al. (2022). Malondialdehyde-Acetaldehyde Extracellular Matrix Protein Adducts Attenuate Unfolded Protein Response During Alcohol and Smoking–Induced Pancreatitis. Gastroenterology. 163(4): 1064-1078.e10.
- Sato-Yamada Y, et al (2022). A SARM1-mitochondrial feedback loop drives neuropathogenesis in a Charcot-Marie-Tooth disease type 2A rat model. J Clin Invest. 132(23):e161566.
- Bautista G, et al (2022). Smooth muscle cell Piezo1 modulates small bowel contractility. Research Square[https://doi.org/10.21203/rs.3.rs-1399324/v1]
- Li Y, et al (2022). A novel recessive mutation in OXR1 is identified in patient with hearing loss recapitulated by the knockdown zebrafish. Human. Molecular Genetics, 32 (5), 764-772
- Isaac CV, et al (2022). A method for the development of cranial fracture histology slides, J Forensic Sci. 00:1–8.
- Soontarapornchai K, et al (2021). Pharmacodynamic Effects of Standard versus High Caffeine Doses in the Developing Brain of Neonatal Rats Exposed to Intermittent Hypoxia. Int. J. Mol. Sci. , 22(7): 3473
- Kim HY, et al (2021). YG-1 Extract Improves Acute Pulmonary Inflammation by Inducing Bronchodilation and Inhibiting Inflammatory Cytokines. Nutrients, 13(10): 3414
- Tropea MR, et al (2021). Genetic deletion of α7 nicotinic acetylcholine receptors induces an age-dependent Alzheimer’s disease-like pathology. Progress in Neurobiology, 206: 102154
- Yan T, et al (2021). St. John’s Wort alleviates dextran sodium sulfate‐induced colitis through pregnane X receptor‐dependent NFκB antagonism. The FASEB Journal,35(11): e21968
- Manlapaz‐Mann A, et al (2021). Effects of omega 3 polyunsaturated fatty acids, antioxidants and/or non‐steroidal inflammatory drugs in the brain of neonatal rats exposed to intermittent hypoxia. International Journal of developmental Neuroscience, 81(5): 448-460.
- Wang S,et al (2021). Moderate tibial loading and treadmill running, but not overloading, protect adult murine bone from destruction by metastasized breast cancer. Bone, 153: 116100.
- Song HK,et al (2021). Alpinia officinarum water extract inhibits the atopic dermatitis-like responses in NC/Nga mice by regulation of inflammatory chemokine production. Biomedicine & Pharmacotherapy, 144: 112322.
- Kruse RL, et al (2021),Endoscopic-mediated, biliary hydrodynamic injection mediating clinically relevant levels of gene delivery in pig liver. Gastrointestinal Endoscopy. 94: 1119-1130.e4.
- Baeet D, et al. CKD-506: A novel HDAC6-selective inhibitor that exerts therapeutic effects in a rodent model of multiple sclerosis. Scientific reports, 11:14466.
- Soontarapornchai K, et al (2021). Pharmacodynamic Effects of Standard versus High Caffeine Doses in the Developing Brain of Neonatal Rats Exposed to Intermittent Hypoxia. Int. J. Mol. Sci. 22(7): 3473.
- Kaczanowska S, et al (2021). Genetically engineered myeloid cells rebalance the core immune suppression program in metastasis. Cell, 184 ( 8): 2033-2052.e21.
- Lee CL, et al (2021). Characterization of cardiovascular injury in mice following partial-heart irradiation with clinically relevant dose and fractionation. Radiotherapy and Oncology.157:155-162.
- Manlapaz-Mann A, et al (2021). Effects of omega 3 polyunsaturated fatty acids, antioxidants, and/or non-steroidal inflammatory drugs in the brain of neonatal rats exposed to intermittent hypoxia. Intl J Dev Neuroscience. 81(5):448-460.
- Ko KW, et al (2021) . integrated Bioactive Scaffold with Polydeoxyribonucleotide and Stem-Cell-Derived Extracellular Vesicles for Kidney Regeneration. ACS Nano; 15 ( 4): 7575–7585.
- Tropea MR, et al (2021). A failure of β-amyloid physiological function due to genetic deletion of α7 nicotinic acetylcholine receptors induces an Alzheimer’s disease-like pathology, BioRxiv. https://doi.org/10.1101/2021.01.05.425382.
- Han Y, et al (2021).Identification of SARS-CoV-2 inhibitors using lung and colonic organoids. Nature; 589: 270–275.
- Diana M, et al (2020). Allograft inflammatory factor-1 in myeloid cells drives autoimmunity in type 1 diabetes. JCI Insight. 5(10): e136092.
- Kawakami M, et al (2020). A Novel CDK2/9 Inhibitor CYC065 Causes Anaphase Catastrophe and Represses Proliferation, Tumorigenesis and Metastasis in Aneuploid Cancers. Mol Cancer Ther. 20(3): 477-489
- Wu B, et al. (2020). Genetic ablation of adipocyte PD-L1 reduces tumor growth but accentuates obesity-associated inflammation. J Immunother Cancer. 8(2): e000964.
- Shen DF, et al.(2020). Astaxanthin suppresses endoplasmic reticulum stress and protects against neuron damage in Parkinson’s disease by regulating miR-7/SNCA axis. Neurosci Res. S0168-0102(20): 30018-3.
- Chen J, et al. (2020). Conventional Co-Housing Modulates Murine Gut Microbiota and Hematopoietic Gene Expression.Int. J. Mol. Sci. 21(17): 6143.
- Diana M, et al. (2020). Allograft inflammatory factor-1 in myeloid cells drives autoimmunity in type 1 diabetes. JCI Insight. 5(10): e136092.
- Li J, et al. (2020). Attenuation of immune‐mediated bone marrow damage in conventionally housed mice. Molecular Carcinogenesis, 59 (2): 237-245.