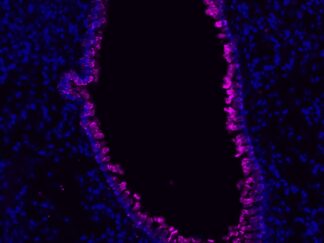
Description
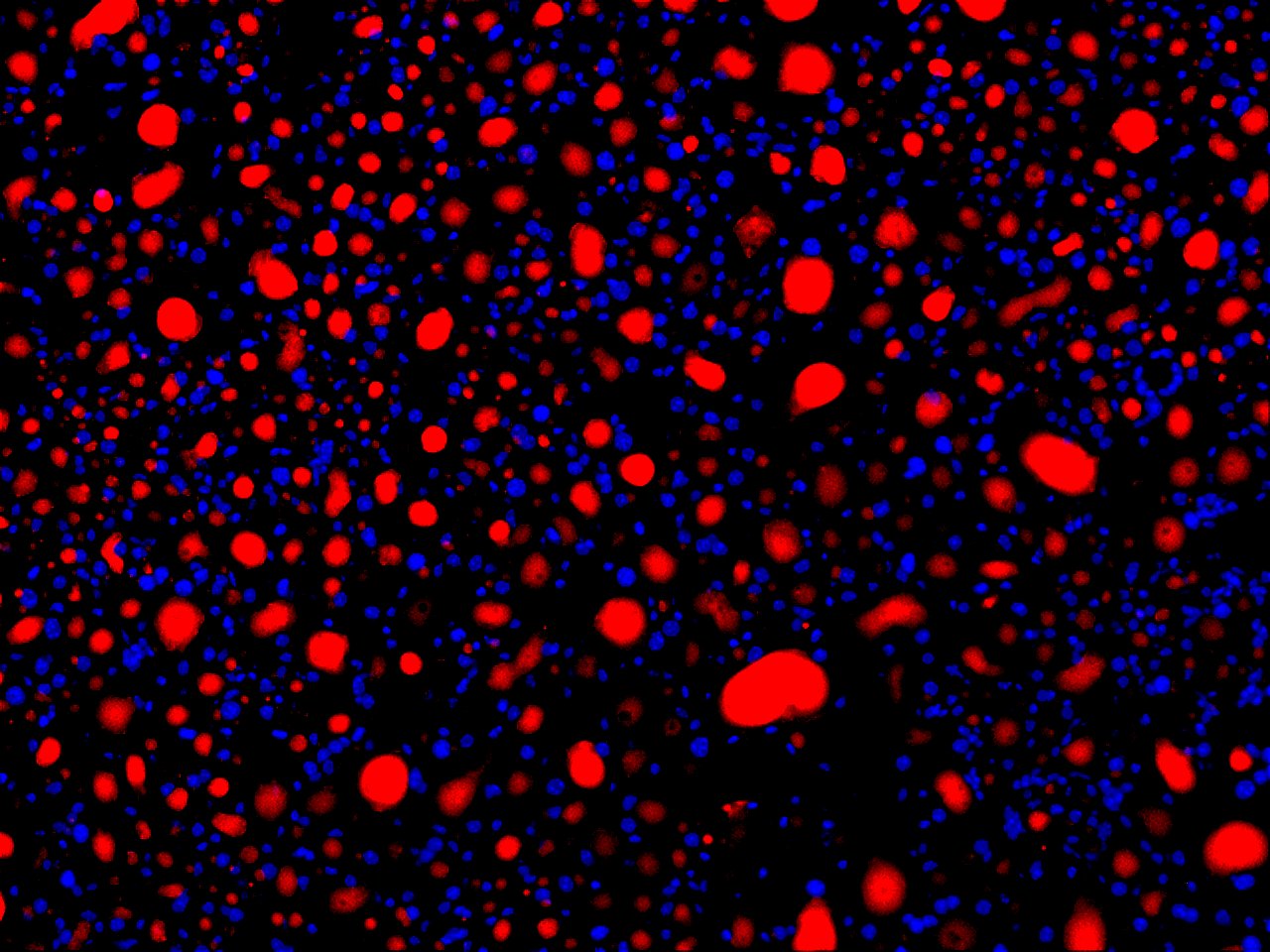
Nile Red is a highly sensitive fluorescent dye used for the detection of intracellular lipid droplets and neutral lipid accumulation in cells and tissues. As a lipophilic stain, Nile Red exhibits strong fluorescence when partitioned into lipid-rich environments, making it a valuable tool for studying lipid metabolism, storage disorders, and cellular responses to metabolic stress.
VitroView™ Nile Red Stain Kit provides a convenient and reliable method for staining both live and fixed cells to visualize and quantify neutral and polar lipids. Upon excitation, Nile Red emits distinct fluorescence spectra depending on the lipid environment—neutral lipids fluoresce yellow-gold, while polar lipids exhibit red fluorescence. This property allows researchers to differentiate between lipid classes within the same sample using standard fluorescence or confocal microscopy.
The kit offers ready-to-use reagents and a streamlined protocol optimized for consistent and reproducible results. It is compatible with a wide range of sample types, including mammalian cells, yeast, bacteria, and tissue sections. The Nile Red Stain Kit is ideal for applications in cell biology, lipidomics, toxicology, and metabolic research.
Kit Contents
| SKU# | Reagent Name | Volume |
| VB-3042-1 | Nile Red Stock Solution | 50µl |
| VB-3042-2 | Staining Buffer | 40ml |
Storage Condition
Store at -20°C and Protect from light.
Staining Procedures
Preparation of Nile Red Working Stain Solution
Dilute the Nile Red stock solution in Staining Buffer immediately before use: 2 µL stock solution in 1 mL Staining Buffer
** Always prepare the working stain solution fresh before each experiment.
Frozen Tissue Samples
- Section Preparation: Cut 5-10 µm thick sections on a cryostat and mount on slides.
- Air Drying: Allow sections to air-dry for a maximum of 15-20 minutes at room temperature to prevent lipid deformation.
- Fixation: Fix the sections in 10% formalin in PBS for 10-15 minutes at room temperature.
Note: Avoid fixatives that remove lipids, such as 100% ethanol, methanol, or acetone, as this will dissolve the very lipids you are trying to stain.
- Washing: Wash the tissue sections gently three times for 5 minutes each with PBS.
- Staining: Stain the samples in the Nile Red working solution (100µl/section) for 10-60 minutes at room temperature in the dark.
- Washing: Gently wash the tissue sections in PBS once for 5 minutes to remove excess dye.
- Counterstain/Mounting: (Optional) Incubate with DAPI solution (e.g., 1:4000 dilution in PBS) for 10-20 minutes to stain nuclei, followed by washing. Mount the sections with an aqueous-based mounting medium (e.g., VitroView™ Anti-Fade Aqueous Mounting Medium or VitroView™ Anti-Fade Permanent Aqueous Mounting Medium) and add a coverslip. Some protocols image directly in a PBS bath using a water-immersion objective without a coverslip/mounting media.
- Imaging/ Slide Storage: observe the cells under a fluorescence microscope with a TRITC filter set. Image immediately or store slides at 4°C, protected from light.
Key Considerations
- Nile Red Fluorescence: Nile Red is intensely fluorescent in hydrophobic environments, staining neutral lipids (triglycerides, cholesteryl esters) yellow-gold and more polar lipids (phospholipids) red. The specific emission can be tuned by the filter set used (e.g., FITC filter set for yellow-gold/neutral lipids).
- Lipid Preservation: The key to frozen section staining is to avoid organic solvents like pure ethanol or acetone during fixation or staining, as they will extract the lipids from the tissue.
- Optimization: Staining duration and concentration may require optimization for different tissue types and thicknesses.
Adherent Cells
- Grow cells in either a 96-well black-wall/clear-bottom plate (100 µL/well) or on coverslips placed in a Petri dish containing the appropriate culture medium.
- Gently aspirate the culture medium and add an equal volume (e.g., 100 µL/well) of the Nile Red Staining Solution.
- Incubate the cells at 37°C in a 5% CO₂ incubator for 10–30 minutes.
- Remove the Nile Red Staining Solution by aspiration.
*Note: Because Nile Red exhibits minimal fluorescence in aqueous media, removal of the culture medium (Step 2) and staining solution (Step 4) after incubation is optional.
- Measure fluorescence at Ex/Em = 550/640 nm using a microplate reader, or observe the cells under a fluorescence microscope with a TRITC filter set.
Suspension Cells
- Centrifuge the cells at 1000 rpm for 5 minutes to obtain 1–5 × 10⁵ cells per tube.
- Resuspend the pellet in 500 µL of Nile Red Staining Solution.
- Incubate at room temperature or 37°C for 10–30 minutes, protected from light.
- Centrifuge to remove the staining solution, then resuspend the cells in 500 µL of pre-warmed medium or buffer to achieve 1–5 × 10⁵ cells per tube.
💡 Note: Since Nile Red has minimal fluorescence in aqueous media, removal of the staining solution after incubation is optional.
- Analyze fluorescence using a fluorescence microscope (TRITC filter set) or a flow cytometer (FL1 channel).
💡 Note: Stained cells can be fixed with 3–4% formaldehyde. Prefixed cells (3–4% formaldehyde) may also be stained with the Nile Red Staining Solution.
Notes
-
-
- Prepare the Nile Red working solution fresh each time for consistent results.
- Protect all samples from light—Nile Red is highly photolabile.
- Avoid using detergents or high ethanol concentrations prior to staining, as these may extract cellular lipids.
- For quantitative analysis, maintain consistent staining, incubation, and imaging parameters across all samples.
-
Expected Results
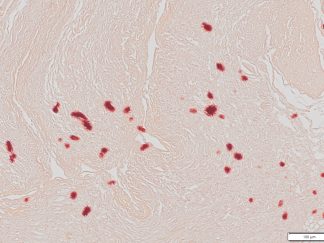
- Calcium salts———Black or brown-black
- Nuclei——————Red
- Cytoplasm————Pink
More Images
User Manual and Material Safety Data Sheet (MSDS) (PDF)
VB-3042 MSDS