Description
The VitroView™ PAS-Diastase (PAS-D) Stain Kit is designed for the detection of glycogen, mucins, and basement membranes in tissue sections. This method combines Periodic Acid-Schiff (PAS) staining with diastase (α-amylase) digestion to specifically remove glycogen, rendering it PAS-negative while other PAS-reactive materials remain stained.
PAS-Diastase stain application:
1. Glycogen Detection:
The PAS stain binds to glycogen, which contains a high amount of polysaccharides (carbohydrates). However, the glycogen can sometimes appear falsely positive because other carbohydrates also react with the PAS reagent.
To overcome this, the diastase (which digests glycogen) is applied before staining. The glycogen in the tissue will be digested by the diastase enzyme and won’t react with the PAS stain. This leaves only the non-glycogen carbohydrate structures to be stained by PAS, making it easy to distinguish between glycogen and other carbohydrates.
2. Histopathological Use:
The PAS-D stain is valuable in identifying glycogen accumulation in tissues, such as in conditions like Glycogen Storage Diseases (GSDs) and certain tumors (e.g., glycogen-rich tumors).
It is also used to identify fungi (as they have polysaccharides in their cell walls), basement membranes, and certain types of mucopolysaccharides in tissues.
3. Differentiating Between Glycogen and Other Substances:
By using PAS-D, pathologists can clearly distinguish between glycogen and other carbohydrate-containing structures because glycogen will not stain after diastase digestion, whereas other structures like mucins, fungal organisms, and basement membranes will still be stained.
Kit Contents
| SKU Number | Component | Volume |
| VB-3045-1 | Diastase Solution | 250 ml |
| VB-3045-2 | Periodic Acid Solution | 250 ml |
| VB-3045-3 | Schiff’s Reagent | 250 ml |
| VB-3045-4 | Mayer’s Hematoxylin Solution | 250 ml |
Storage Condition:
Diastase Solution, Periodic Acid Solution and Schiff’s Reagent: Store at 2–8°C.
Mayer’s Hematoxylin Solution: Store at room temperature.
Protocol for FFPE Tissue Sections
- Immerse sections in Xylene for 6 minutes (twice).
- Immerse in 100% Ethanol for 2 minutes.
- Immerse in 95% Ethanol for 2 minutes (twice).
- Immerse in 70% Ethanol for 2 minutes.
- Rinse sections in distilled water for 5 minutes.
- Treat sections with warm Diastase Solution at 37°C for 20-30 minutes in an incubator or water bath.
- Wash sections thoroughly in running tap water.
- Incubate sections with Periodic Acid Solution for 5 minutes.
- Rinse sections in distilled water.
- Cover sections with Schiff’s Reagent in a dark staining chamber for 5-15 minutes.
- Wash sections in fast-running tap water for 3–5 minutes to develop the magenta color.
- Counterstain with Mayer’s Hematoxylin Solution for 1 minute.
- Rinse sections in tap water for 5 minutes.
- Dehydrate sections using 3 changes of xylene (5 minutes per change).
- Mount sections with Permount or another suitable organic mounting medium
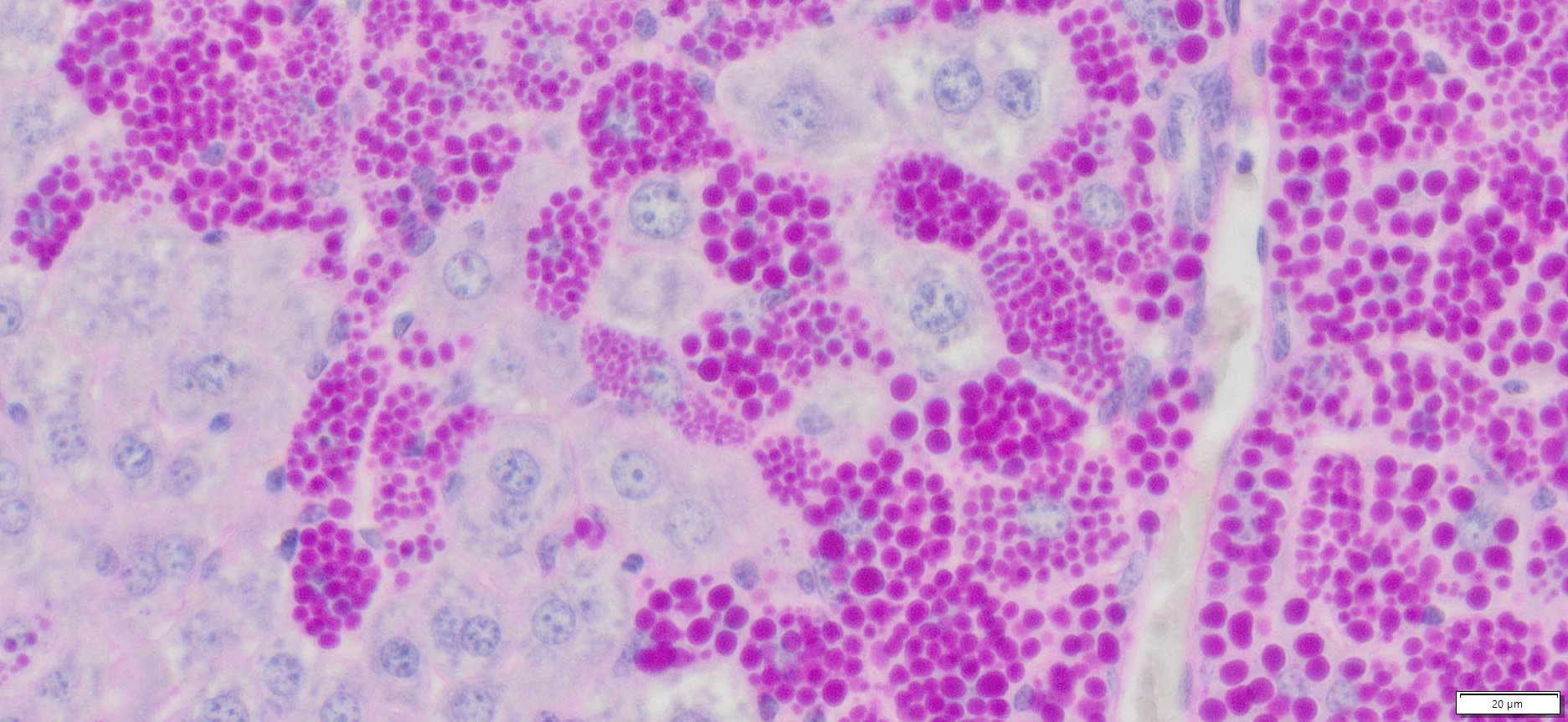
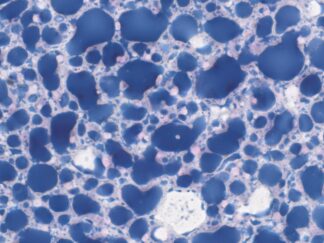
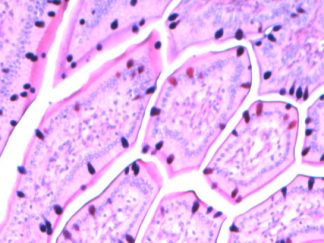
Expected Results
- Mucins—————————–Magenta
- Nuclei——————————Dark Blue
- Glycogen————————–Not stained (glycogen is removed by diastase digestion)
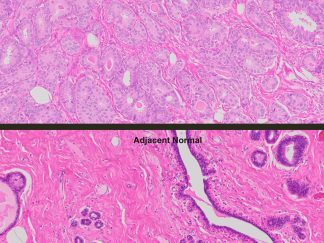
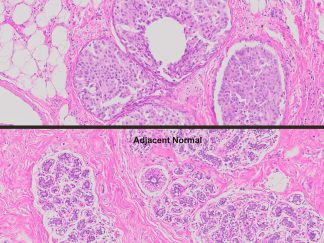
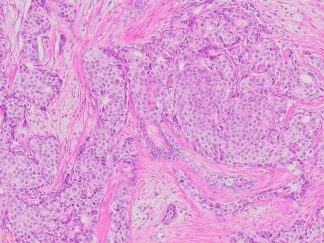
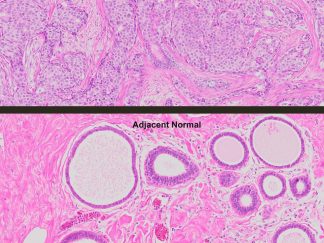
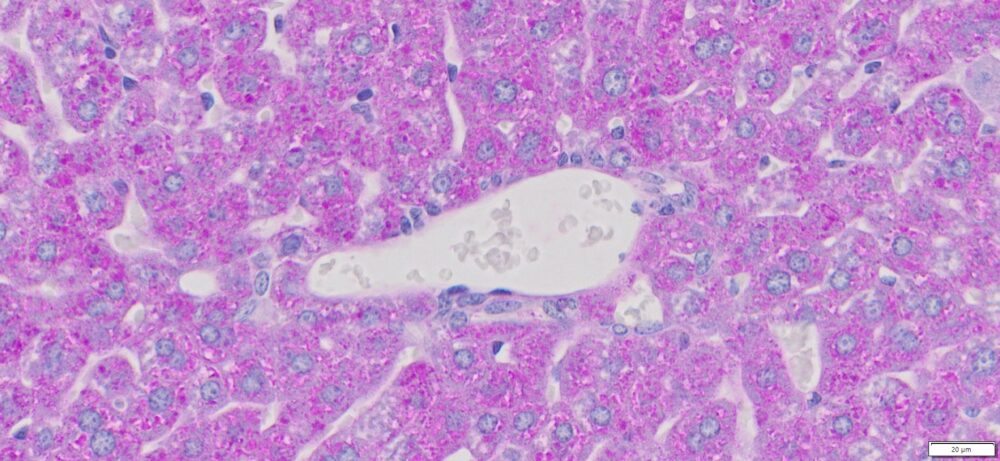
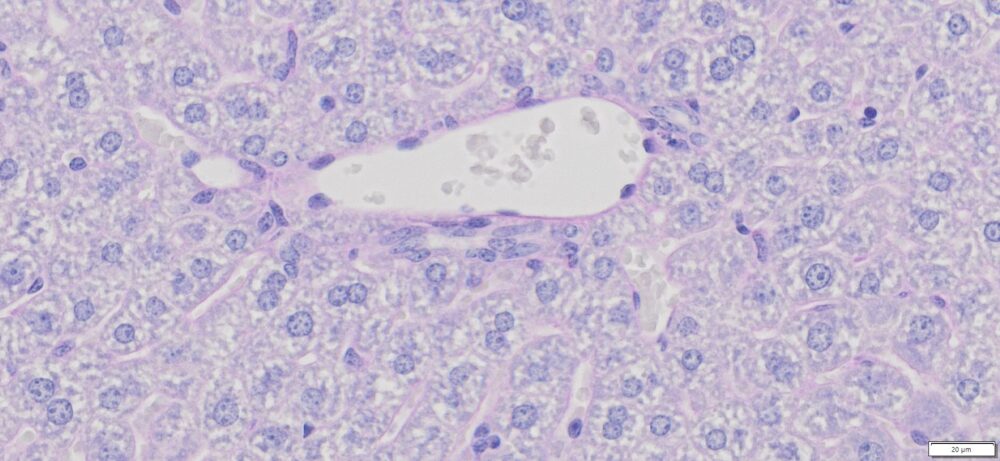
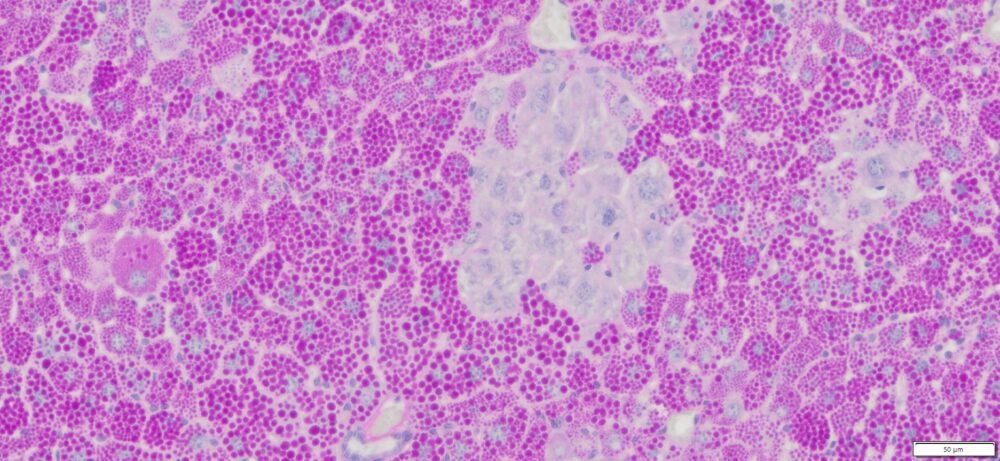
More PAS-Diastase (PAS-D) Staining Images
Precautions:
- Handle reagents with care.
- Avoid contact with eyes, skin, or clothing.
- Do not ingest.
- Always wear gloves when handling chemicals.