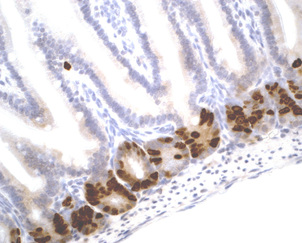
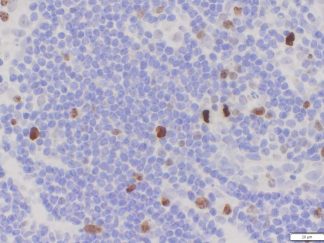
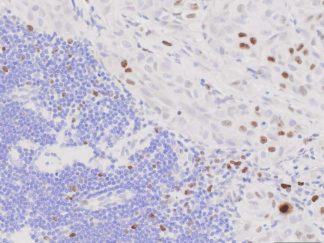
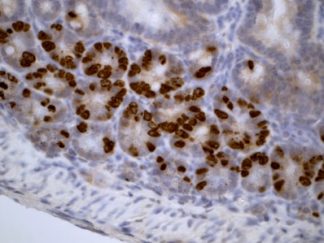
Description
Cell proliferation is an important and highly studied cellular function. 5-bromo-2’-deoxyuridine (BrdU) can be incorporated into the newly synthesized DNA of replicating cells (during the S phase of the cell cycle), substituting for thymidine during DNA replication. BrdU is commonly used in the detection of proliferating cells and label-retaining cells in living tissues. VitroView In Situ BrdU IHC Detection Kit detects BrdU incorporated into cellular DNA during cell proliferation using an anti-BrdU antibody. This kit is designed for immunohistochemical staining of BrdU in formalin-fixed paraffin-embedded (FFPE) sections, frozen sections, and cultured or isolated cells on slides. Our antibody against BrdU works in all species tested (human, mouse, and rat) with strong specific staining and minimal background.
Application
BrdU Immunohistochemistry for in situ detecting cell proliferation in FFPE, frozen section and cultured cells on slides from all species.
Contents
| VB-4001-1 | 10×BrdU Retrieval Solution | 50ml |
| VB-4001-2 | RTU Block buffer | 5ml |
| VB-4001-3 | RTU anti-BrdU Antibody | 5 ml |
| VB-4001-4 | RTU polymeric peroxidase conjugated secondary antibody | 5 ml |
| VB-4001-5 | BrdU Positive Control FFPE Slides | 2 slides |
Note: RTU=ready-to-use
Reagents and Material Required but Not Provided
- BrdU labeling reagent
- Xylene and ethanol
- Distilled or deionized water
- 30% hydrogen peroxide
- 10 mM phosphate-buffered saline (PBS), pH 7.4
- Triton X-100
- Mini PAP Pen
- DAB Substrate Kit (Cat#: VB-6003 or VB-6003E)
- Hematoxylin (Cat#:VB-6004)
- Mounting Media
Storage Condition
Store at 2-8°C.
Protocol
1.BrdU labeling
Prior to BrdU immunostaining for detection of proliferating cells, BrdU need to be labeled in the living cells or tissues. Labeling can be done either in vitro or in vivo depend on the purpose of scientific research. Below is several protocols for BrdU labeling.
- In vitro BrdU Labeling of Proliferating Cells
To label cultured tissues or cells, carefully add BrdU to culture medium at a final concentration of 3μg/ml. For this step, do not disturb the cells in any way that may disrupt their normal cell cycling patterns. Incubate culture cells/tissue at 37º C for 0.5~4 hrs. The incubation time with BrdU is dependent on the test cell population’s rate of cell cycle entry and progression. Cells from the same population that are not BrdU-labeled are the negative cell staining control.
- In vivo Labeling of Mouse Proliferating Cells
Make a working solution of BrdU in PBS at 10mg/ml. One to four hours before scarifying, mice are given intraperitoneally 70 mg/kg of body weight BrdU. Incorporation of BrdU can be detected in thymus and bone marrow in as little as 1 hr post injection. At 24 hr post injection BrdU can be detected in most tissues.
- In vivo Labeling for Mouse Label-retaining Cells
For continuous labeling in vivo, BrdU (50 mg/ml) is administered to mice throughout a 7-day period via a subcutaneous miniosmotic pump (Alzet model 2001, Durect Corporation, Cupertino, CA). Alzet pumps are implanted in mice and removed after one week. Or dilute BrdU to 0.8 mg/ml in the drinking water. For long-term studies, feeding mice with BrdU for 9 consecutive days, followed by a changeover to normal water, has worked effectively.Mice are sacrificed 4~10 weeks after removal of the Alzet pumps or a changeover to normal water after 9 day BrdU drinking.
2. Preparation of Slides
1) Cell Lines
- BrdU labeled cells are cultured on sterile glass cover slips or slides at 37 º C
- Wash briefly with PBS
- Fix in ice cold acetone or ice cold methanol for 10 minutes. Air dries for 30 minutes.
- Wash in PBS
- Follow procedure for BrdU antigen retrieval as required.
2) Frozen Sections
- Snap frozen fresh tissues in liquid nitrogen or isopentane pre-cooled in liquid nitrogen, embedded in OCT compound in cryomolds. Store the frozen tissue block at -80°C until ready for sectioning.
- Transfer the frozen tissue block to a cryotome cryostat (e.g. -20°C) prior to sectioning and allow the temperature of the frozen tissue block to equilibrate to the temperature of the cryotome cryostat.
- Section the frozen tissue block into a desired thickness (typically 5-10 µm) using the cryotome.
- Place the tissue sections onto glass slides suitable for immunohistochemistry.
- Sections can be stored in a sealed slide box at -80°C for later use.
- Before staining, warm slides at room temperature for 30 minutes and fix in ice cold acetone or ice cold methanol for 10 minutes.
- Air dry for 30 minutes.
- Wash in PBS
- Follow procedure for BrdU antigen retrieval as required.
3) Paraffin Sections
- Deparaffinize sections in xylene, 3×5min.
- Hydrate with 100% ethanol, 2×2min.
- Hydrate with 95% ethanol, 2×2min.
- Rinse in distilled water.
- Follow procedure for BrdU antigen retrieval as required.
3. BrdU Antigen Retrieval
Prepare 100ml of 1×BrdU retrieval working solution by mixing 10 ml of 10× BrdU Retrieval Solution with 90 ml of ddH2O. Place the slides in a coplin jar containing the BrdU retrieval working solution and heat in a microwave oven for 3 min to bring slides to boil. Other sources of heat such as pressure cooker or hot water bath may be used. Maintain at a sub-boiling temperature for 10 minutes. Slowly cool slides on bench top for 30 minutes.
4. Staining Procedure
- Rinse with PBST for 3min×2 and in PBS for 3min×2.
- Incubate sections with RTU Block buffer for 15min at RT in humidified chamber.
- Remove blocking buffer, add 2~3 drops of RTU anti-BrdU antibody and incubate at 4°C for overnight or at RT for 1 hour in humidified chamber, then wash with PBS for 3min×2
- Peroxidase Blocking (optional): incubate sections in 0.3% hydrogen peroxide in PBS for 10 minutes at room temperature. Rinse in PBS.
- Detection: incubate sections with 3-4 drops of RTU polymeric peroxidase conjugated secondary antibody for 30 minutes at room temperature.
- Rinse in PBS for 3×2min.
- Chromogen/Substrate: incubate sections with 3 drops of DAB solution for 2-8 minutes. Monitor signal development under a microscope. Note: DAB solution is made by mixture of 25 µl of DAB stock solution with 1ml of DAB enhancer buffer (dark-brown stain) or DAB buffer (brown stain) which are included in DAB Substrate Kit (SKU#: VB-6003 or VB-6003E).
- Rinse in distilled water 2×2 min.
- Counterstain: For using Hematoxylin Nuclear Counterstaining Kit (SKU#: VB-6004), incubate sections with 3 drops of RTU hematoxylin solution for 1-2 minutes. Rinse in tape water 2×2 min.
- Dehydrate through 75% ethanol for 2 min, 95% ethanol for 2 min, and 100% ethanol for 2x3min. Clear in xylene for 2×5 min.
- Coverslip with mounting medium.
Warning: DAB is a possible carcinogen. Please take necessary precautions.
Note: This product is intended for research purposes only. This product is not intended to be used for therapeutic or diagnostic purposes in humans or animals. Avoid contact with eyes, skin and clothing. Do not ingest. Wear gloves.
References
- Medina Benavente JJ, Mogami H, Sakurai T, Sawada K. Evaluation of silicon nitride as a substrate for culture of PC12 cells: an interfacial model for functional studies in neurons. PLoS One. 2014 ,9(2):e90189.
- Jensen-Taubman S, Wang XY, Linnoila RI. Achaete-scute homologue-1 tapers neuroendocrine cell differentiation in lungs after exposure to naphthalene. Toxicol Sci. 2010, 117(1):238-48.
- Tough, D. F., and J. Sprent. Turnover of naive- and memory-phenotype T cells. J. Exp.Med. 1994, 179:1127-1135.
- deFazio A., Leary J. A., Hedley D. W., and Tattersall M. H. Immunohistochemical detection of proliferating cells in vivo. J Histochem Cytochem 1987, 35(5):571-577