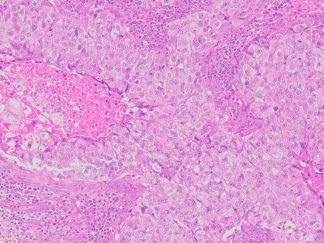
Description
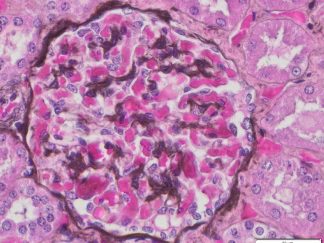
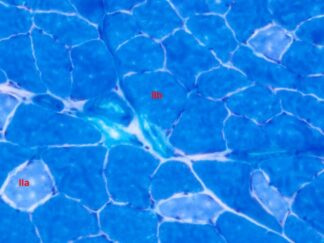
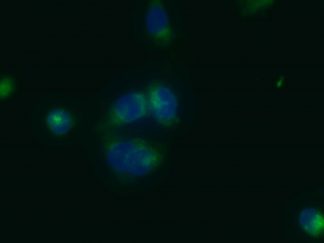
VitroView™ Gelatin-coated microscope slides are specially prepared glass slides with a thin layer of gelatin to enhance adhesion of biological specimens such as tissue sections or cells. These slides are ideal for histology, immunohistochemistry, and cytology applications.
Advantage
- Improved tissue adhesion
- Ideal for Thin or delicate sections
- Compatibility with aqueous Stains
- Minimized background interference
- Biocompatible surface
- Frosted write-on end
Package Size
- 25 ×75×1 mm, 25 slides/box.
- Slides are packaged in a high-quality slide box, sealed in an airtight plastic bag to maintain dryness during shipping and storage
Storage Conditions
- Store in a cool, dry place at 15–25°C (59–77°F)
- Avoid direct sunlight and moisture
- Keep slides in a dust-free slide box or container
- Use slides within 3–6 months for optimal adhesion
Handling Instructions
- Handle slides by the edges to avoid fingerprints on the coated surface
- Wear powder-free gloves to maintain cleanliness
- Use clean, dry forceps when placing slides onto holders or trays
Preparation Before Use
- Labeling: Use a solvent-resistant pen to label slides on the frosted end. Allow ink to dry before proceeding
- Warming (optional): Pre-warm slides to 37–40°C in an incubator or warming plate if using with paraffin-embedded sections
Mounting Tissue Sections
Float Sections:
- Cut tissue sections using a microtome (e.g., 4–200 µm)
- Float sections on a warm water bath (40–45°C)
- Transfer to Slide: Use forceps to place tissue section on the gelatin-coated side
- Flatten the section using gentle heat if needed
Dry Slides:
- Dry slides horizontally at 37–42°C for 30 minutes to overnight
- Ensure slides are completely dry before further staining
Staining Compatibility
Gelatin-coated slides are compatible with most staining protocols, including:
- Hematoxylin and Eosin (H&E)
- Immunohistochemistry (IHC)
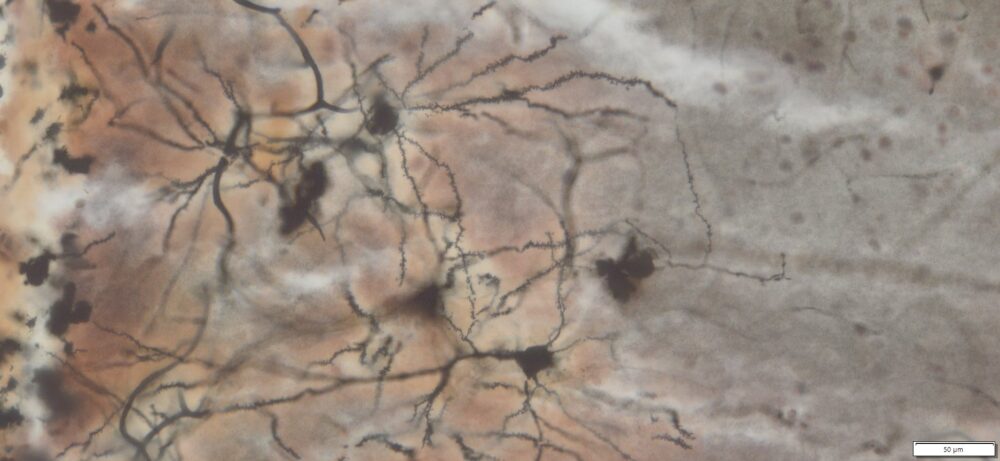
- Special stains: Golgi-Cox, PAS, and trichrome staining, ect.
Note: Avoid excessive agitation or prolonged incubation in harsh solvents, which may reduce tissue adherence.
Troubleshooting
| Problem | Cause | Solution |
| Tissue falls off | Incomplete drying or poor adhesion | Ensure proper drying at 37–42°C |
| Background staining | Excess gelatin or poor rinsing | Rinse slides in distilled water before use |
| Air bubbles | Poor tissue placement | Gently flatten sections on water bath |
Disposal Instructions
- Dispose of used slides in designated sharps containers
- Follow institutional or local biohazard waste protocols if slides are exposed to biological materials
Safety Information
- Gelatin is non-toxic but handle all biological materials as potentially infectious
- Use PPE: lab coat, gloves, and eye protection
- Use slides in a well-ventilated lab or under a fume hood if applying chemicals