Description
VitroView™ Human on Human (HOH) IHC Kit is specifically designed for the use of human (or humanized) primary antibodies on frozen or paraffin-embedded human tissue sections. This kit overcomes the common challenge of high background staining caused by endogenous human immunoglobulin.
Key Advantages:
- Low background with significant reduction of endogenous human IgG staining
- High sensitivity with excellent signal-to-noise ratio
- Fewer steps and reduced protocol time
- Ready-to-use reagents for ease of application
Application:
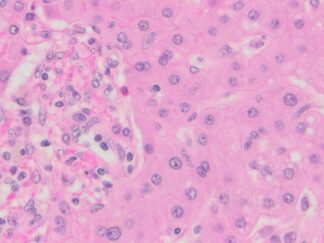
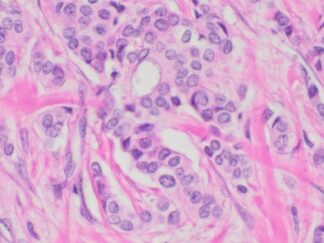
Immunohistochemistry for detecting a primary human antibody on human tissue sample.
Contents
| RTU HOH Protein blocking solution | 10ml x 2 |
| RTU human IgG blocking solution | 10 ml |
| RTU biotinylated anti-human secondary antibody | 10 ml |
| RTU streptavidin-HRP | 10 ml |
| DAB stock solution (40×) | 0.75 ml |
| Stable DAB buffer | 30 ml |
| RTU hematoxylin solution | 10 ml |
Note: RTU=ready-to-use
Reagents and Material Required but Not Provided
- Xylene and ethanol
- Distilled or deionized water
- 30% hydrogen peroxide
- 10 mM phosphate-buffered saline (PBS), pH 7.4
- Triton X-100
- Mini PAP Pen
- Primary antibody
- Mounting Media
Storage Condition
Store at 2-8°C.
Protocol
- Preparation of Slides
- For Cell Lines
- Grow cultured cells on sterile glass cover slips or slides overnight at 37 º C
- Wash briefly with PBS
- Fix as desired. Possible procedures include: a) 20 minutes with 10% formalin in PBS (keep wet); 2) 10 minutes with ice cold methanol, allow to air dry; 3) 10 minutes with ice cold acetone, allow to air dry
- Wash in PBS
For Frozen Sections
- Snap frozen fresh tissues in liquid nitrogen or isopentane pre-cooled in liquid nitrogen, embedded in OCT compound in cryomolds. Store the frozen tissue block at -80°C until ready for sectioning.
- Transfer the frozen tissue block to a cryotome cryostat (e.g. -20°C) prior to sectioning and allow the temperature of the frozen tissue block to equilibrate to the temperature of the cryotome cryostat.
- Section the frozen tissue block into a desired thickness (typically 5-10 µm) using the cryotome.
- Place the tissue sections onto glass slides suitable for immunohistochemistry (e.g. Superfrost).
- Sections can be stored in a sealed slide box at -80°C for later use.
- Before staining, warm slides at room temperature for 30 minutes and fix in ice cold acetone or ice cold methanol for 10 minutes. Air dry for 30 minutes.
- Wash in PBS
For Paraffin Sections
- Deparaffinize sections in xylene, 3×5min.
- Hydrate with 00% ethanol, 2×2min.
- Hydrate with 95% ethanol, 2×2min.
- Rinse in distilled water.
- Follow procedure for pretreatment as required.
- Antigen retrieval
Most formalin-fixed tissue requires an antigen retrieval step before immunohistochemical staining can proceed. Heat-mediated and enzymatic antigen retrievals are common methods.
- For Citrate: Bring slides to a boil in 10 mM sodium citrate buffer, pH 6.0; maintain at a sub-boiling temperature for 10 minutes. Cool slides on bench top for 30 minutes.
- For EDTA: Bring slides to a boil in 1 mM EDTA, pH 8.0: follow with 15 minutes at a sub-boiling temperature. No cooling is necessary.
- For TE: Bring slides to a boil in 10 mM TE/1 mM EDTA, pH 9.0: then maintain at a sub-boiling temperature for 18 minutes. Cool at room temperature for 30 minutes.
- For Pepsin: Digest for 10 minutes at 37°C.
Note: Do not use this pretreatment with frozen sections or cultured cells that are not paraffin-embedded.
- Staining Procedure
- Rinse sections in PBS-Triton X-100 (0.025%) for 2×2min.
- Serum Blocking: incubate sections with 2-4 drops of RTU HOH protein blocking solution for 30 minutes at room temperature to block non-specific binding of immunoglobulin.
- Human IgG Blocking: Mouse IgG Blocking: Incubate tissue sections with 2–4 drops of RTU human IgG blocking solution for 60 minutes at room temperature or overnight at 4°C to effectively block endogenous mouse immunoglobulins. After incubation, rinse sections with PBS.
- Primary Antibody: Incubate sections with primary antibody (human IgG) at an appropriate dilution in RTU HOH protein blocking solution for 30-60 min at room temperature.
Note: With HOH IHC, the primary antibody incubation is often shorter than usual (e.g., 30 minutes to 1 hour). Overnight incubation can sometimes increase the background.
- Peroxidase Blocking: incubate sections in 0.3% hydrogen peroxide in PBS for 10 minutes at room temperature. Rinse in PBS.
- Secondary Antibody: incubate sections with 2-4 drops of RTU biotinylated anti-human secondary antibody for 30 minutes at room temperature. Rinse in PBS for 3×2min.
- Detection: incubate sections with 2-4 drops of RTU Streptavidin-HRP for 30 minutes at room temperature. Rinse in PBS for 3×2min.
- Chromogen/Substrate: incubate sections with 2-4 drops of DAB solution for 2-8 minutes. Monitor signal development under a microscope. Rinse in distilled water 2×2 min.
Note: DAB solution is made by mixture of 25 µl of DAB stock solution with 1ml of DAB buffer.
- Counterstain: Incubate sections with 3 drops of RTU hematoxylin solution for 1-2 minutes. Rinse in tape water 2×2 min.
- Dehydrate by 75% ethanol for 2 min, 95% ethanol for 2 min, and 100% ethanol for 2x3min. Clear in xylene for 2×5min.
- Mount a coverslip onto a glass slide with Permount or some other suitable organic mounting medium.
User Manual ans MSDS (PDF)