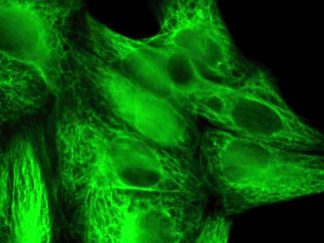
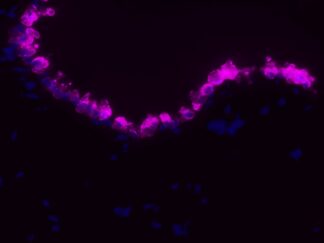
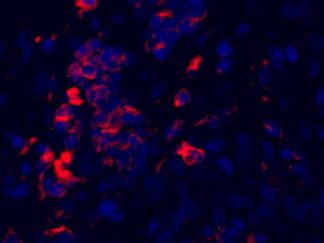
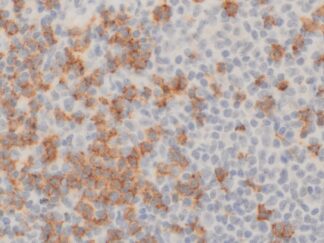
Description
VitroView™ FL594 Conjugated Goat Anti–Mouse IgG Secondary Antibody is exceptionally well-suited for a wide range of applications, including fluorescence microscopy, confocal laser scanning microscopy, flow cytometry, and fluorescent western detection. Our extensive selection of fluorescent markers allows for the precise customization of our reagents to seamlessly integrate with virtually any fluorescent detection system.
These Goat Anti–Mouse IgG antibody conjugates are meticulously prepared from affinity-purified antibodies that specifically target IgG heavy chains and all classes of immunoglobulin light chains found in rabbits. To minimize cross-reactivity, our goat anti–mouse IgG antibodies are subjected to adsorption against human IgG and human serum prior to the conjugation process.
Each conjugate typically carries 2–8 fluorophores per IgG molecule, ensuring robust labeling. Prior to distribution, our products are thoroughly quality-certified to guarantee the absence of unconjugated dyes and undergo comprehensive immunofluorescence testing to confirm their minimal nonspecific staining characteristics.
Specification
| Physical State | 1.0 mg/ml |
| Purification | Immunoaffinity chromatography |
| Buffer | PBS, pH 7.4 |
| Perservative | 0.02% Sodium azide |
| Stabilizer | 0.1% BSA |
| Host/Isotype | Goat |
| Class&Form | Polyclonal/Whole Antibody |
| Type | Secondary Antibody |
| Tested Species | Mouse |
| Target Class | IgG(H+L) |
| Excitation/Emission | 590/617 nm |
Package Size:
200µg
Reconstitution and Storage
Product is stable for about 6 months at 2- 8ºC as an undiluted liquid. Prepare working dilution fresh each day. For extended storage after rehydration, add an equal volume of glycerol for a final concentration of 50%, and store at -20 ºC as a liquid.
Guidelines for Use
Centrifuge the reconstituted protein conjugate solution briefly in a microcentrifuge before use. Add only the supernatant to the experiment. This step eliminates any protein aggregates that may have formed during storage, thereby reducing nonspecific background staining. Because staining protocols vary with application, the appropriate dilution of antibody should be determined empirically. For the fluorophore-labeled antibodies, a final concentration of 1–10 μg/ml should be satisfactory for most immunohistochemical applications. For flow cytometry applications, 0.06–1.0 μg per 1 × 106 cells should yield satisfactory result.
General Protocols
For Cultured Cells
- Grow cultured cells on sterile glass cover slips or slides overnight at 37 º C.
- Wash briefly with PBS.
- Fix as desired. Possible procedures include: a) 20 minutes with 10% formalin in PBS (keep wet); 2) 10 minutes with ice cold methanol, allow to air dry; 3) 10 minutes with ice cold acetone, allow to air dry.
- Wash in PBS.
For Frozen Sections
- Snap frozen fresh tissues in liquid nitrogen or isopentane pre-cooled in liquid nitrogen, embedded in OCT compound in cryomolds. Store the frozen tissue block at -80°C until ready for sectioning.
- Transfer the frozen tissue block to a cryotome cryostat (e.g. -20°C) prior to sectioning and allow the temperature of the frozen tissue block to equilibrate to the temperature of the cryotome cryostat.
- Section the frozen tissue block into a desired thickness (typically 5-10 µm) using the cryotome.
- Place the tissue sections onto glass slides suitable for immunohistochemistry (e.g. Superfrost).
- Sections can be stored in a sealed slide box at -80°C for later use.
- Before staining, warm slides at room temperature for 30 minutes and fix in ice cold acetone or ice cold methanol for 10 minutes. Air dry for 30 minutes.
- Wash in PBS.
For paraffin sections
- Deparaffinize sections in xylene, 3×5min.
- Hydrate with 00% ethanol, 2×2min.
- Hydrate with 95% ethanol, 2×2min.
- Rinse in distilled water.
- Follow procedure for pretreatment as required.
- Antigen retrieval
Most formalin-fixed tissues requires an antigen retrieval step before immunohistochemical staining can proceed. Heat-mediated and enzymatic antigen retrievals are common methods.
- For Citrate: Bring slides to a boil in 10 mM sodium citrate buffer, pH 6.0; maintain at a sub-boiling temperature for 10 minutes. Cool slides on bench top for 30 minutes.
- For EDTA: Bring slides to a boil in 1 mM EDTA, pH 8.0: follow with 15 minutes at a sub-boiling temperature. No cooling is necessary.
- For TE: Bring slides to a boil in 10 mM TE/1 mM EDTA, pH 9.0: then maintain at a sub-boiling temperature for 18 minutes. Cool at room temperature for 30 minutes.
- For Pepsin: Digest for 10 minutes at 37°C.0
Note: Generally it is not necessary to pretreat frozen sections or cultured cells with above antigen retrieval protocol.
- Staining Procedure
- Rinse sections in PBS-Triton X-100 (0.025%) for 2×2min.
- Serum Blocking: incubate sections with 2-4 drops of RTU normal goat serum for 30 minutes to block non-specific binding of immunoglobulin.
- Primary Antibody: incubate sections with primary antibody (mouse IgG) at appropriate dilution in antibody dilution buffer (SKU#: VB-6002) for 1-2 hour at room temperature or overnight at 4 °C. Rinse in PBS.
- Detection: To initiate the detection process, incubate the sections with a working dilution of VitroView™ FL594 Conjugated Goat Anti–Mouse IgG Secondary Antibody , typically requiring 2-4 drops, for duration of 30 minutes at room temperature. Rinse in PBS for 3×2min.
- Count stain with Dapi (blue fluorescence) or other fluorescence nuclei stain dye (blue or green color).
- Mount with aqueous mounting medium.
- The slides can be visualized under fluorescence microscope using the correct filter.
- Store slides in the dark at 4°C.
Staining Protocol for Flow Cytometry
There are many alternative procedures that can be used for specific staining experiments. The protocol below is a general guideline for flow cytometry and should be optimized or modified for each application.
- Aliquot 1× 106 cells into 12 × 75 mm polypropylene tubes for flow cytometry.
- For intracellular staining, cells can be fixed first to ensure stability of soluble antigens or antigens with short half-lives. We recommend a fix and perm kit from reliable manufacturers. Follow manufacturer’s instructions.
- Add the primary antibody or isotype control at the appropriate dilution to the assay tubes. Incubate according to manufacturer’s instructions.
- Rinse cells twice by centrifugation with 2-3 ml incubation buffer.
- Decant supernatant and re-suspend the pellet in remaining volume of wash.
- Add fluorescent secondary antibody and incubate for 20-30 minutes. General range for secondary antibodies is between 1-10 μg/ml for IgG conjugates for most applications.
- Rinse cells twice by centrifugation with 2-3 ml incubation buffer. Centrifuge to collect cells after each wash. Decant supernatant.
- Resuspend cells in 0.5 ml of diluent of choice to analyze on flow cytometer. Acquire data using the correct channel.