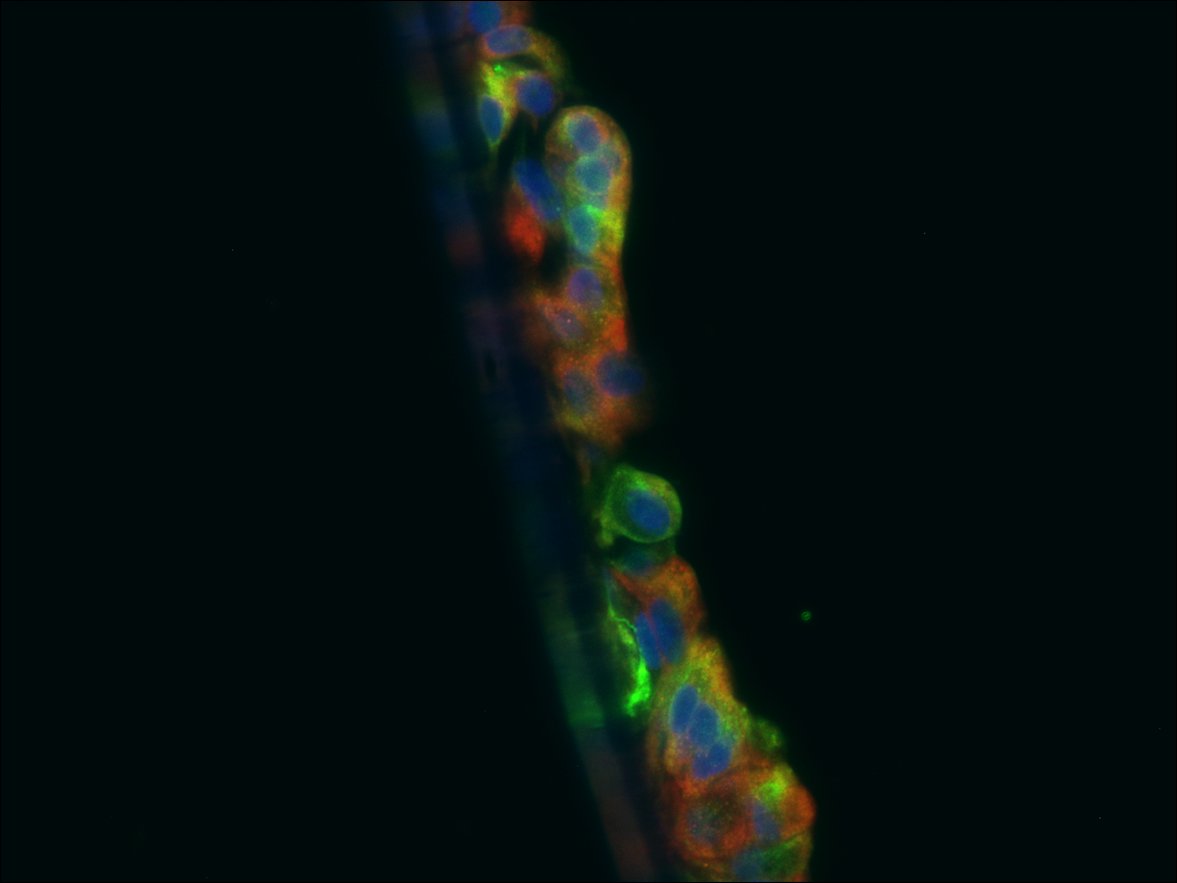
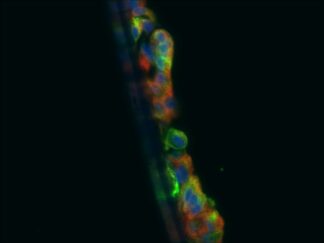
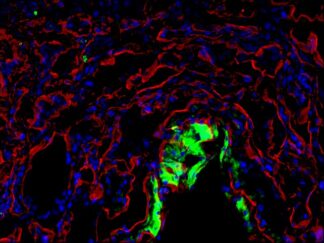
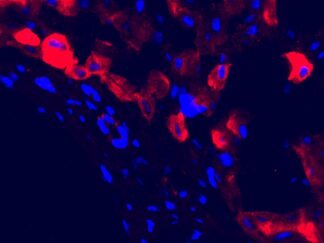
Description
A double immunofluorescence (IF) procedure is needed in order to be able to examine the co-distribution of two different antigens in the same sample.
The advantages of this kit: 1) High sensitivity; 2) Low background; 3) Reduction of steps and time; 4) Simplified double labeling.
Specifications
| Unit Size | 1 kit |
| Target Species | Mouse and Rat |
| Color of Fluorescence | FL488 Conjugated Goat anti Mouse IgG (Green)
FL594 Conjugated Goat anti Rat IgG (Red) |
| Excitation/Emission for FL488 dye | 496/519 nm |
| Excitation/Emission for FL594 dye | 590/617 nm |
| Host Species | Goat |
| Application | Double IF staining for simultaneous detection of primary antibodies raised in mouse and rat |
Contents
RTU Normal Goat Serum ————————————————————-10ml
FL488 Conjugated Goat anti Mouse IgG (1mg/ml)————————–50µl
FL594 Conjugated Goat anti Rat IgG (1mg/ml)—————————–50µl
Aqueous Anti-fade Mounting Medium with Dapi———————- 1.5 ml×2
Note: RTU=ready-to-use
Reagents and Material Required but Not Provided
-
- Xylene and ethanol
- Distilled or deionized water
- 10 mM phosphate-buffered saline (PBS), pH 7.4
- Triton X-100
- Mini PAP Pen
- Primary antibody
- Antibody Dilution Buffer (SKU#: VB-6002)
- BSA
Storage Condition
Store at 2-8°C.
Protocol
1. Preparation of Slides
A. Cell Lines
- Grow cultured cells on sterile glass cover slips or slides overnight at 37°C.
- Briefly wash with PBS.
- Choose one of the following fixation methods (To achieve optimal results, it may be necessary to optimize the fixation methods):
a. Fix in 10% formalin in PBS for 20 minutes (keep wet).
b. Immerse in ice-cold methanol for 10 minutes and allow to air dry.
c. immerse in ice-cold acetone for 10 minutes and allow to air dry.
- Wash slides in PBS.
B. Frozen Sections
- Snap-freeze fresh tissues in liquid nitrogen or isopentane pre-cooled in liquid nitrogen, then embed them in OCT compound in cryomolds. Store the frozen tissue block at -80°C until ready for sectioning.
- Before sectioning, equilibrate the frozen tissue block to the temperature of the cryotome cryostat (e.g., -20°C).
- Section the frozen tissue block into the desired thickness (typically 5-10 µm) using the cryotome.
- Place the tissue sections onto glass slides suitable for immunohistochemistry (e.g., Superfrost).
- Store sections in a sealed slide box at -80°C for later use.
- Before staining, allow slides to warm to room temperature for 30 minutes and then fix in ice-cold acetone or ice-cold methanol for 10 minutes. Air dry for 30 minutes.
- Wash in PBS.
C. Paraffin Sections
- Deparaffinize sections in xylene, 3×5 minutes.
- Hydrate with 100% ethanol, 2×2 minutes.
- Hydrate with 95% ethanol, 2×2 minutes.
- Rinse in distilled water.
- Follow the required pretreatment procedure.
2. Antigen Retrieval
- Most formalin-fixed tissues require antigen retrieval before proceeding with immunohistochemical staining. Common methods include heat-mediated and enzymatic antigen retrievals.
- Choose the appropriate method:
a. For Citrate: Bring slides to a boil in 10 mM sodium citrate buffer, pH 6.0, and maintain at a sub-boiling temperature for 10 minutes. Cool slides on the benchtop for 30 minutes.
b. For EDTA: Bring slides to a boil in 1 mM EDTA, pH 8.0, and follow with 15 minutes at a sub-boiling temperature. No cooling is necessary.
c. For TE: Bring slides to a boil in 10 mM TE/1 mM EDTA, pH 9.0, and then maintain at a sub-boiling temperature for 18 minutes. Cool at room temperature for 30 minutes.
d. For Pepsin: Digest for 10 minutes at 37°C.
Note: Do not use this pretreatment with frozen sections or cultured cells that are not paraffin-embedded.
3. Staining Procedure
- Rinse sections in PBS-Triton X-100 (0.025%) for two 2-minute cycles.
- Serum Blocking: Incubate sections with 2-4 drops of ready-to-use (RTU) normal goat serum for 30 minutes to block non-specific immunoglobulin binding.
- Primary Antibody: Incubate sections with a mixture of two primary antibodies (mouse and rat IgG) at the appropriate dilution in antibody dilution buffer (SKU# #: VB-6002) for 1-2 hours at room temperature or overnight at 2-8°C.
- Rinse in PBS.
- Detection: Incubate sections with a mixture (70-150 µl) of two secondary antibodies raised in different species (with two fluorochromes including anti-mouse FL488 and anti-rat FL594 secondary antibodies) at appropriate dilutions (1/100-1/500) in 0.1% BSA in PBS for 1 hour at room temperature in the dark.
- Rinse in PBS for three 2-minute cycles.
- Counterstaining and mount cover slip with 20-30µl of aqueous anti-fade mounting medium with DAPI. Seal cover slip with nail polish to prevent drying and movement.
- Visualize and capture the signal using a fluorescence microscope equipped with excitation wavelength filters of 488 nm and 594 nm.
- Store slides in the dark at 2-8°C.
More Images
User Manual and Material Safety Data Sheet (MSDS) (PDF)
Note
This product is intended for research purposes only. This product is not intended to be used for therapeutic or diagnostic purposes in humans or animals.
Precautions: Handle with care. Avoid contact with eyes, skin and clothing. Do not ingest. Wear gloves.