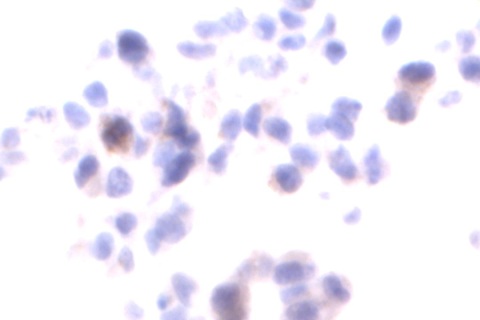
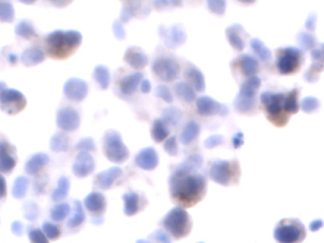
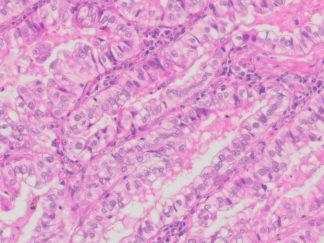
Description
Autophagy (or autophagocytosis) is the basic catabolic mechanism that involves cell degradation of unnecessary or dysfunctional cellular components through the actions of lysosomes. Transmission electron microscopies (TEM) as well as immunohistochemistry are indispensable tools for the evaluation of autophagy in situ. LC3B is currently considered as one of the most reliable markers of the autophagic process. The in situ autophagy LC3B IHC-DAB detection kit has been optimized for the detection of the autophagy marker protein LC3B in paraffin-embedded tissue specimens, cultured cells and frozen sections. The antibody against LC3B works in human, mouse, and rat with strong specific staining and lower background.
Application
In situ detection of autophagy cells in in human, mouse, and rat cells/tissues
Contents
| VB-4004D-1 | 10×Antigen retrieval solution | 50 ml |
| VB-4004D-2 | RTU Block buffer | 5 ml |
| VB-4004D-3 | RTU anti-LC3B antibody | 5 ml |
| VB-4004D-4 | RTU Polymeric Peroxidase Conjugated Secondary Antibody | 5 ml |
| VB-4004D-5 | DAB Stock Solution (40×) | 0.5 ml |
| VB-4004D-6 | Stable DAB Buffer | 15 ml |
| VB-4004D-7 | LC3B positive control FFPE slides | 2 slides |
Note: RTU=ready-to-use
Reagents and Material Required but Not Provided
- Xylene and ethanol
- Distilled or deionized water
- 30% hydrogen peroxide
- 10 mM phosphate-buffered saline (PBS), pH 7.4
- Triton X-100
- Mini PAP Pen
- Hematoxylin (Cat#:VB-6004)
- Mounting Media
Storage Condition
Store at 2-8°C.
Protocol
1. Preparation of Slides
- For Cell Lines
- Grow cultured cells on sterile glass cover slips or slides overnight at 37 º C
- Wash briefly with PBS
- Fix as desired. Possible procedures include: a) 20 minutes with 10% formalin in PBS (keep wet); or b) 10 minutes with ice cold methanol, allow to air dry; or c) 10 minutes with ice cold acetone, allow to air dry
- Wash in PBS
For Frozen Sections
- Snap frozen fresh tissues in liquid nitrogen or isopentane pre-cooled in liquid nitrogen, embedded in OCT compound in cryomolds. Store the frozen tissue block at -80°C until ready for sectioning.
- Transfer the frozen tissue block to a cryotome cryostat (e.g. -20°C) prior to sectioning and allow the temperature of the frozen tissue block to equilibrate to the temperature of the cryotome cryostat.
- Section the frozen tissue block into a desired thickness (typically 5-10 µm) using the cryotome.
- Place the tissue sections onto glass slides suitable for immunohistochemistry (e.g. Superfrost).
- Sections can be stored in a sealed slide box at -80°C for later use.
- Before staining, warm slides at room temperature for 30 minutes and fix in ice cold acetone or ice cold methanol for 10 minutes. Air dry for 30 minutes.
- Wash in PBS
For Paraffin Sections
- Deparaffinize sections in xylene, 3×5min.
- Hydrate with 100% ethanol, 2×2min.
- Hydrate with 95% ethanol, 2×2min.
- Rinse in distilled water.
- Follow procedure for pretreatment as required.
2. Antigen retrieval
Formalin-fixed tissues require an antigen retrieval step before immunohistochemical staining can proceed.
- Dilute 10×Ag Retrieval Solution to 1×Ag Retrieval Solution by adding 90ml ddH2O into 10ml of 10×Ag Retrieval Solution.
- Bring slides to a boil in 1×Ag retrieval solution at a sub-boiling temperature for 10-15 minutes. Cool slides on bench top for 30 minutes.
Note: Do not use this pretreatment with frozen sections or cultured cells that are not paraffin-embedded.
3. Staining Procedure
- Rinse sections in PBS-Triton X-100 (0.025%) for 2×2min
- Serum Blocking: incubate sections with 2-3 drops of RTU normal goat serum for 30 minutes to block non-specific binding of immunoglobulin.
- Primary Antibody: incubate sections with RTU anti-LC3B primary antibody at room temperature for 1-2 hour or at 4 °C for overnight. Rinse in PBS.
- Peroxidase Blocking (optional): incubate sections in 0.3% hydrogen peroxide in PBS for 10 minutes at room temperature. Rinse in PBS.
- Detection: incubate sections with 3-4 drops of RTU polymeric peroxidase conjugated secondary antibody for 30 minutes at room temperature.
- Rinse in PBS for 3×2min.
- Chromogen/Substrate: incubate sections with 3 drops of DAB solution for 2-8 minutes. Monitor signal development under a microscope. Note: DAB solution is made by mixture of 25 µl of DAB stock solution with 1ml of DAB enhancer buffer (dark-brown stain) or DAB buffer (brown stain) which are included in DAB Substrate Kit (SKU#: VB-6003 or VB-6003E).
- Rinse in distilled water 2×2 min.
- Counterstain: For using Hematoxylin Nuclear Counterstaining Kit (SKU#: VB-6004), incubate sections with 3 drops of RTU hematoxylin solution for 1-2 minutes. Rinse in tape water 2×2 min.
- Dehydrate through 75% ethanol for 2 min, 95% ethanol for 2 min, and 100% ethanol for 2x3min. Clear in xylene for 2×5 min.
- Coverslip with mounting medium.