Description
VitroView™ Mouse on Mouse IHC Kit is specifically designed for applications involving mouse primary antibodies on mouse tissue samples. It is ideal for use in transgenic and knockout mouse models, as well as in multiple antigen staining protocols, allowing the detection of two distinct mouse primary antibodies on the same tissue section using either fluorescent or enzyme-based detection systems.
Key Advantages:
- Biotin-free system eliminates biotin-related background issues
- Low background with significant reduction of endogenous mouse IgG staining
- High sensitivity with excellent signal-to-noise ratio
- Fewer steps and shorter protocol time
- Ready-to-use reagents for convenience
Application:
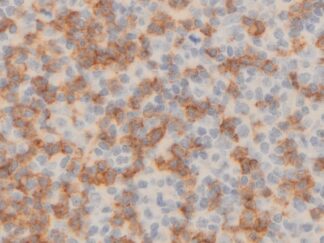
Immunohistochemistry for detecting a primary mouse antibody on mouse tissue sample.
Contents
| RTU MOM Protein blocking solution | 10ml x 2 |
| RTU mouse IgG blocking solution | 10 ml |
| RTU polymeric peroxidase anti-mouse secondary antibody | 10 ml |
| DAB stock solution (40×) | 0.75 ml |
| Stable DAB buffer | 30 ml |
| RTU hematoxylin solution | 10 ml |
Note: RTU=ready-to-use
Reagents and Material Required but Not Provided
- Xylene and ethanol
- Distilled or deionized water
- 30% hydrogen peroxide
- 10 mM phosphate-buffered saline (PBS), pH 7.4
- Triton X-100
- Mini PAP Pen
- Primary antibody
- Mounting Media
Storage Condition
Store at 2-8°C.
Protocol
- Preparation of Slides
- For Cell Lines
- Grow cultured cells on sterile glass cover slips or slides overnight at 37 º C
- Wash briefly with PBS
- Fix as desired. Possible procedures include: a) 20 minutes with 10% formalin in PBS (keep wet); 2) 10 minutes with ice cold methanol, allow to air dry; 3) 10 minutes with ice cold acetone, allow to air dry
- Wash in PBS
For Frozen Sections
- Snap frozen fresh tissues in liquid nitrogen or isopentane pre-cooled in liquid nitrogen, embedded in OCT compound in cryomolds. Store the frozen tissue block at -80°C until ready for sectioning.
- Transfer the frozen tissue block to a cryotome cryostat (e.g. -20°C) prior to sectioning and allow the temperature of the frozen tissue block to equilibrate to the temperature of the cryotome cryostat.
- Section the frozen tissue block into a desired thickness (typically 5-10 µm) using the cryotome.
- Place the tissue sections onto glass slides suitable for immunohistochemistry (e.g. Superfrost).
- Sections can be stored in a sealed slide box at -80°C for later use.
- Before staining, warm slides at room temperature for 30 minutes and fix in ice cold acetone or ice cold methanol for 10 minutes. Air dry for 30 minutes.
- Wash in PBS
For Paraffin Sections
- Deparaffinize sections in xylene, 3×5min.
- Hydrate with 00% ethanol, 2×2min.
- Hydrate with 95% ethanol, 2×2min.
- Rinse in distilled water.
- Follow procedure for pretreatment as required.
- Antigen retrieval
Most formalin-fixed tissue requires an antigen retrieval step before immunohistochemical staining can proceed. Heat-mediated and enzymatic antigen retrievals are common methods.
- For Citrate: Bring slides to a boil in 10 mM sodium citrate buffer, pH 6.0; maintain at a sub-boiling temperature for 10 minutes. Cool slides on bench top for 30 minutes.
- For EDTA: Bring slides to a boil in 1 mM EDTA, pH 8.0: follow with 15 minutes at a sub-boiling temperature. No cooling is necessary.
- For TE: Bring slides to a boil in 10 mM TE/1 mM EDTA, pH 9.0: then maintain at a sub-boiling temperature for 18 minutes. Cool at room temperature for 30 minutes.
- For Pepsin: Digest for 10 minutes at 37°C.
Note: Do not use this pretreatment with frozen sections or cultured cells that are not paraffin-embedded.
- Staining Procedure
- Rinse sections in PBS-Triton X-100 (0.025%) for 2×2min.
- Protein Blocking: incubate sections with 2-4 drops of MOM protein blocking solution for 30 minutes at room temperature to block non-specific binding of immunoglobulin.
- Mouse IgG Blocking: Incubate tissue sections with 2–4 drops of RTU mouse IgG blocking solution for 60 minutes at room temperature or overnight at 4°C to effectively block endogenous mouse immunoglobulins. After incubation, rinse sections with PBS.
- Primary Antibody: incubate sections with primary antibody (mouse IgG) at appropriate dilution in RTU MOM Protein blocking solution for 30-60 min at room temperature.
Note: With M.O.M., the primary antibody incubation is often shorter than usual (e.g., 30 mins to 1 hour). Overnight incubation can sometimes increase background.
- Rinse in PBS for 3×2min.
- Peroxidase Blocking: incubate sections in 0.3% hydrogen peroxide in PBS for 10 minutes at
room temperature. Rinse in PBS.
- Detection: incubate sections with 2-4 drops of RTU polymeric peroxidase anti-mouse secondary antibody for 30 minutes at room temperature. Rinse in PBS for 3×2min.
- Chromogen/Substrate: incubate sections with 3 drops of DAB solution for 2-8 minutes. Monitor signal development under a microscope.
Note: DAB solution is made by mixture of 25 µl of DAB stock solution with 1ml of Stable DAB buffer.
- Rinse in distilled water 2×2 min.
- Counterstain: Incubate sections with 2-4 drops of RTU hematoxylin solution for 1-2 minutes. Rinse in tape water 2×2 min.
- Dehydrate through 75% ethanol for 2 min, 95% ethanol for 2 min, and 100% ethanol for 2x3min. Clear in xylene for 2×5min.
- Mount a coverslip onto a glass slide with Permount or some other suitable organic mounting medium.
User Manual ans MSDS (PDF)