Description
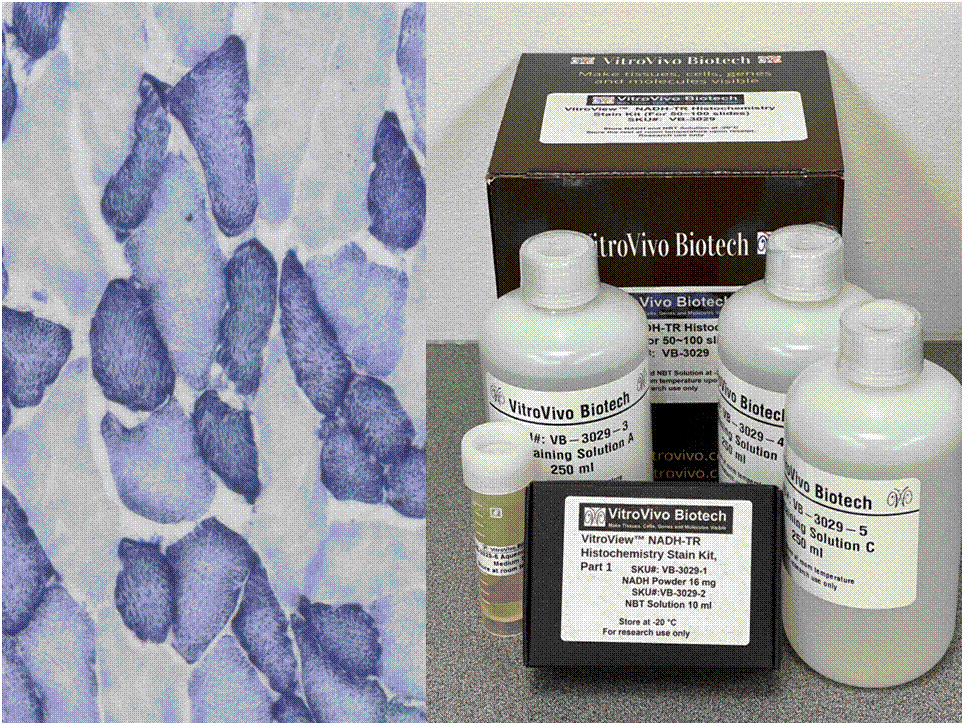
Nicotinamide adenine dinucleotide (NADH) serves as a crucial coenzyme, aiding in substrate reduction reactions linked with glycolysis, oxidative phosphorylation, and fermentation processes. The NADH-TR stain protocol relies on enzymatic activity to liberate hydrogen from NADH, resulting in the formation of a purple-blue formazan pigment, highlighting the sites of reaction.
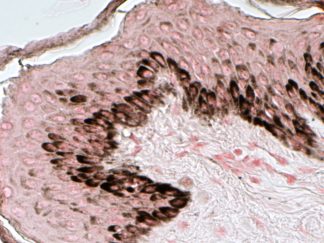
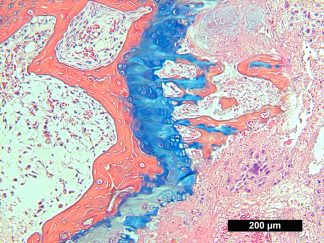
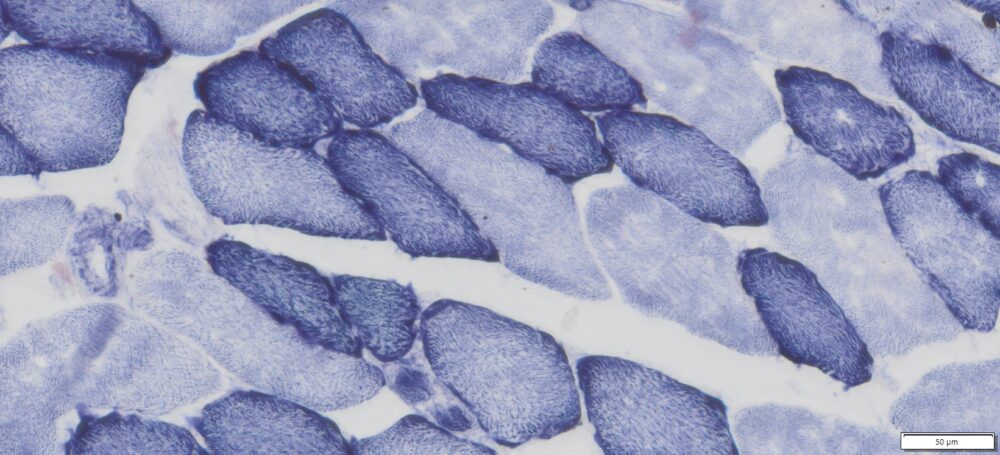
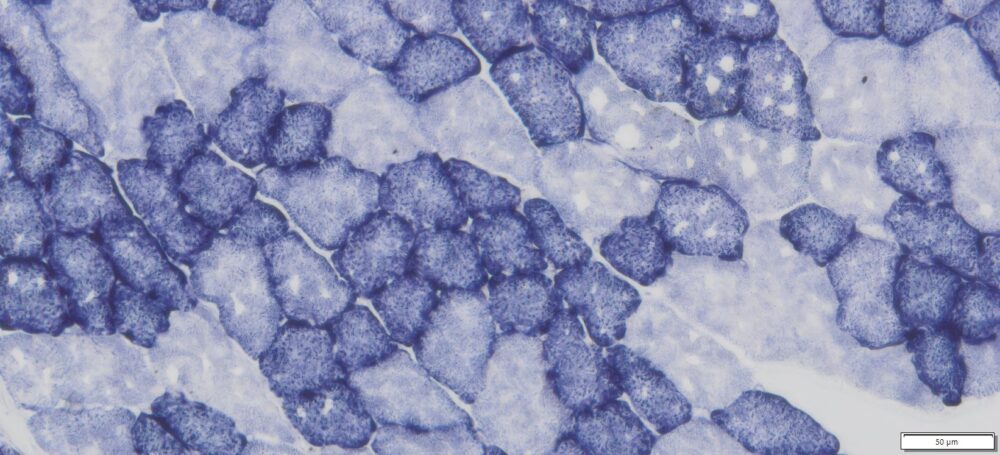
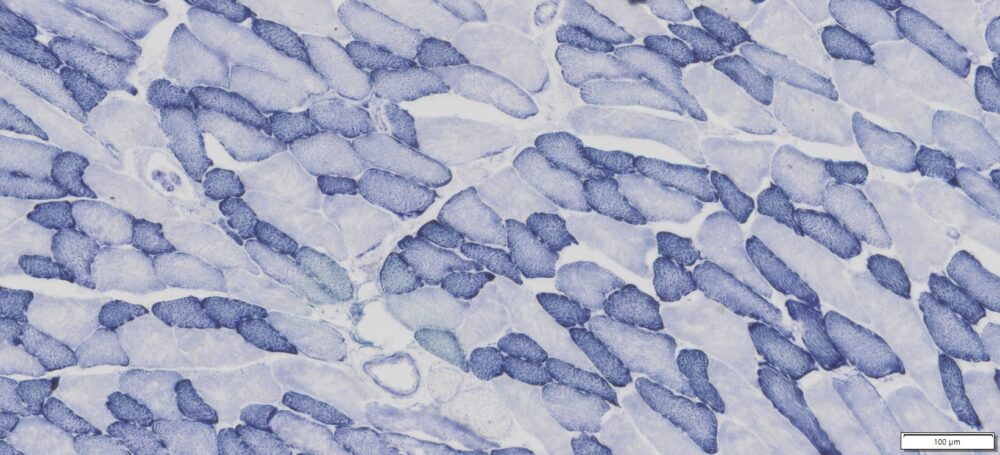
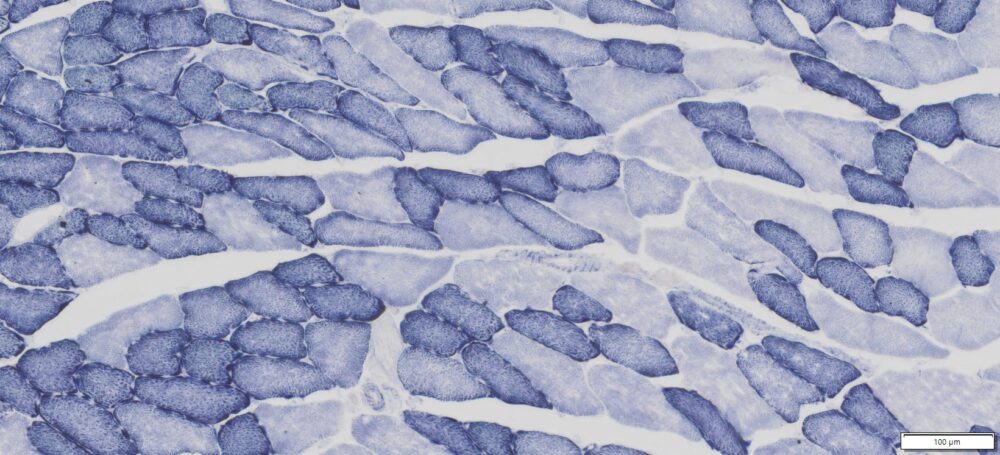
This particular enzymatic histochemical stain using NADH reveals the architectural details of myofibrils, mitochondria, and specific muscle fibers. It plays a significant role in distinguishing between type I (oxidative) and type II (non-oxidative) fibers.
Primarily, this stain kit finds application in demonstrating the patterns of myofiber injury characteristic of congenital and mitochondrial myopathies, as well as specific muscular dystrophies. Notably, this stain protocol necessitates the use of snap-frozen muscle biopsies for its execution.
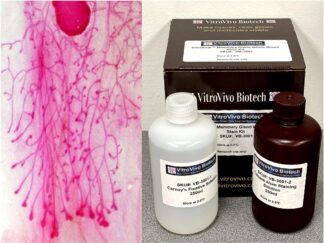
Kit Components
| SKU# | Reagent | Size |
| VB-3029-1 | NADH Powder | 16 mg |
| VB-3029-2 | NBT Solution | 10ml |
| VB-3029-3 | Destaining Solution A | 250 ml |
| VB-3029-4 | Destaining Solution B | 250 ml |
| VB-3029-5 | Destaining Solution C | 250 ml |
| VB-3029-6 | Aqueous mounting medium | 10 ml |
Storage
Store NADH powder and NBT Solution at -20°C.The other components can be stored at room temperature.
Protocol
- Tissue Preparation for Cryosectioning
- Euthanize the animal in compliance with the relevant ethical permit through cervical dislocation or decapitation.
- Collect tissues of interest promptly, without fixation, and rapidly freeze them using dry ice or isopentane, or propane chilled with liquid nitrogen for optimal morphology.
- Store tissues wrapped in aluminum foil at -80°C until ready for sectioning.
- Embed the frozen tissue in preparation for cryosectioning.
- Cut 10-16μm cryostat sections. Thaw sections onto slides at room temperature for 2-5 minutes, and store slides without cover-slipping at -20°C until use.
- NADH Staining
- Preparation of NADH-NBT Solution: Dissolve 16 mg of NADH Powder in 10 ml of NBT Solution. After thorough mixing, aliquot into 2 ml vials and store at -20°C for long-term storage. Label it as NADH-NBT Solution.
- Apply 80~200 μl of NADH-NBT Solution immediately onto frozen sectioned slides in a humidity chamber. Incubate in darkness for 15 minutes at 37°C.
- Remove unbound NBT by performing three exchanges using Destaining Solutions A to C.
- Allow Destaining Solution C to cover the sections until a faint purple cloud is observed over the section.
- Rinse the sections several times with deionized H2O.
- Mount coverslips using an aqueous mounting medium.
Positive Control
Snap frozen striated muscle
Expected Results
- Blue-Purple formazan precipitate is deposited on mitochondria and sarcoplasmic network.
- Type I fibers are darker than type II. Walls of blood vessels also are stained.
- Necrotic muscle fibers lose staining.
More Images

References
-
- Turoczi Z, et al. (2014) Muscle Fiber Viability, a Novel Method for the Fast Detection of Ischemic Muscle Injury in Rats. PLoS ONE 9(1): e84783. doi:10.1371/journal.pone.0084783
- Hebling A, et al. (2009). Muscle fibre types and connective tissue morphometry in frontal muscle of Norfolk rabbits (Oryctolagus cuniculus). Int. J. Morphol., 27(1):187-191
- Nina Hamalxinen and Dirk Pette. (1993). The Histochemical Profiles of Fast Fiber Types IIB, IID,and IIA in Skeletal Muscles of Mouse, Rat, and Rabbit. J Histochem Cytochem. 41(5),733-743
Note
This product is intended for research purposes only. This product is not intended to be used for therapeutic or diagnostic purposes in humans or animals.
Precautions
Handle with care. Avoid contact with eyes, skin and clothing. Do not ingest. Wear gloves.