Description
The nucleus is a membrane-bound organelle found in eukaryotic cells. Cell nuclei contain most of the cell′s genetic material (chromosome), organized as multiple long linear DNA molecules complexed with various proteins, such as histones, to form chromosomes. The nucleus is the primary site of gene expression and DNA replication, within which is a sub-compartment known as the nucleolus responsible for synthesizing rRNA and assembling ribosomes.
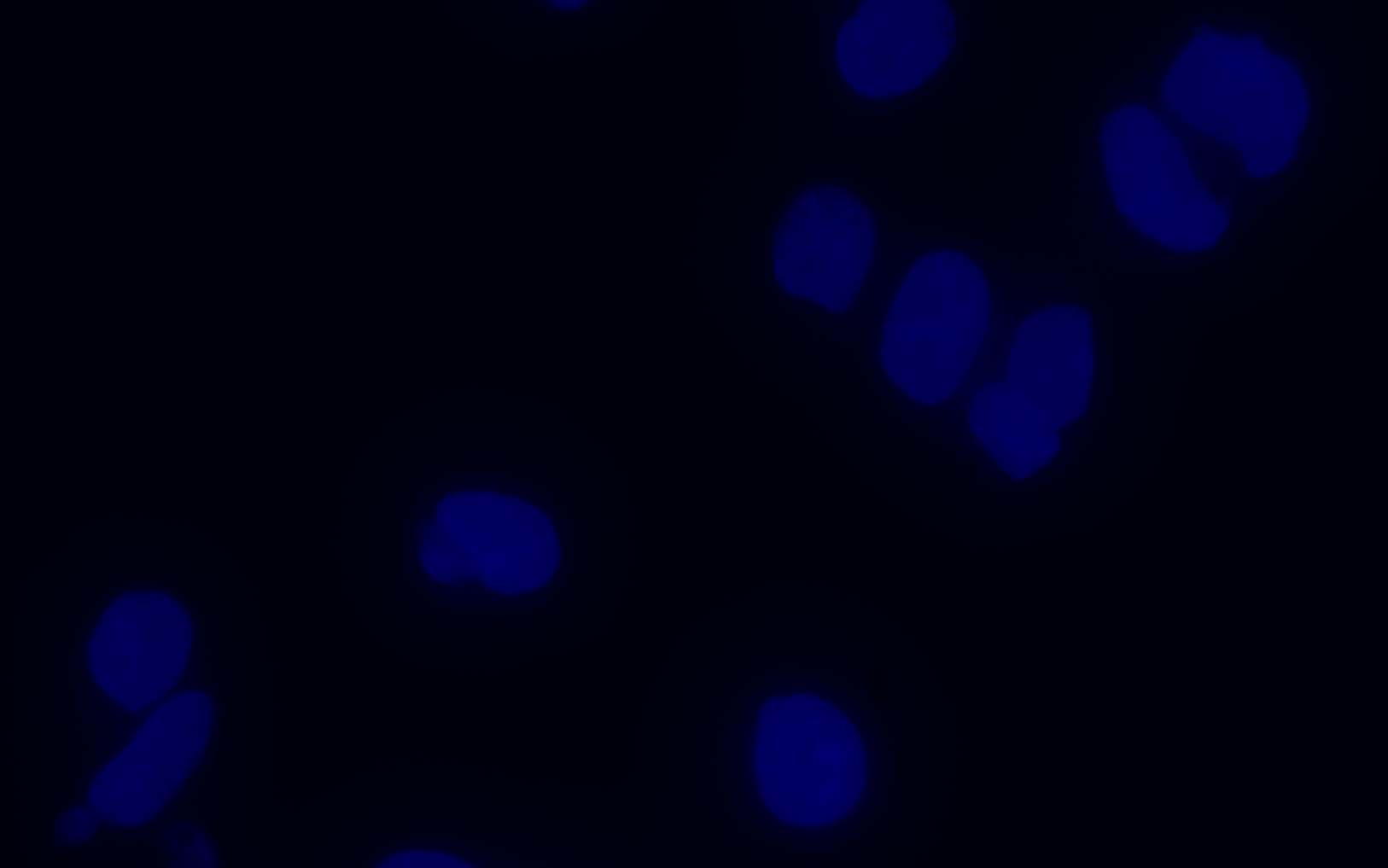
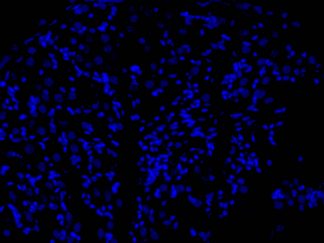
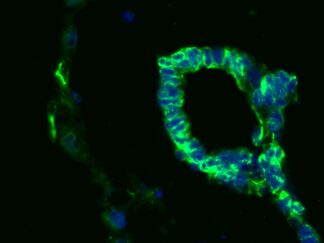
Hoechst 33342 is a popular cell-permeant nuclear counterstain fluorescent dye that emits blue fluorescence (Ex:355nm/Em:465nm) when bound within the minor groove of dsDNA AT-rich regions. This dye can be used in both live and fixed cells
Content
VB-1001 RTU Hoechst 33342 Nuclear Staining Solution——–30 ml
Storage Condition
Store at 2-8 °C and protect from light.
Application
Cell nuclear staining in both live and fixed cells
General Protocol:
Adherent cells for fluorescence microscopy
- Grow cultured cells on sterile glass cover slips or slides overnight at 37 ºC.
- Follow appropriate protocol to fix cultured cells.
- Completely wash the cells with PBS as needed.
- Add adequate RTU Hoechst 33342 Nuclear Staining Solution to cover the whole sample.
- Incubate under dark at room temperature for 5–15 minutes.
- Rinse the sample several times with PBS and remove excess dye.
- Add antifade aqueous mounting medium and mount.
- Use appropriate filters and detect under fluorescence microscope according to standard protocol.
Suspension cells for fluorescence microscopy
- The cells are harvested into a 15 mL polypropylene centrifuge tube and spin down for 8 min at 600 RPM,
- The supernatant is discarded and the cells are resuspended in 0.5 ml of culture medium
- 1-2 drops of the cell suspension were placed on a slide in the central area and moved around to form a thin and even film with a glass spreader.
- (Option) cytocentrifuge can also be used to prepare cell slides.
- Air dry and follow appropriate protocol to fix cultured cells.
- Drop adequate RTU Hoechst 33342 Nuclear Staining Solution to cover the whole sample on slide.
- Incubate under dark at room temperature for 1–5 minutes.
- Cover with coverslip and view under fluorescence microscope according to standard protocol.
For paraffin sections
- Deparaffinize sections in xylene, 3×5min.
- Hydrate with 100% ethanol, 2×2min.
- Hydrate with 95% ethanol, 2×2min.
- Rinse in distilled water.
- Add adequate RTU Hoechst 33342 Nuclear Staining Solution to cover the whole sample.
- Incubate under dark at room temperature for 5–15 minutes.
- Rinse the sample several times with PBS and remove excess dye.
- Add antifade aqueous mounting medium and mount.
- Use appropriate filters and detect under fluorescence microscope according to standard protocol.
For frozen sections
- Before staining, warm frozen slides at room temperature for 30 minutes.
- Fix in ice cold acetone or ice cold methanol for 10 minutes.
- Air dry for 30 minutes and wash in PBS.
- Add adequate RTU Hoechst 33342 Nuclear Staining Solution to cover the whole sample.
- Incubate under dark at room temperature for 5–15 minutes.
- Rinse the sample several times with PBS and remove excess dye.
- Add antifade aqueous mounting medium and mount.
- Use appropriate filters and detect under fluorescence microscope according to standard protocol.
Note: This product is intended for research purposes only. This product is not intended to be used for therapeutic or diagnostic purposes in humans or animals.
References
- Uzunbajakava N, et al. Nonresonant Confocal Raman Imaging of DNA and Protein Distribution in Apoptotic Cells. Biophysical Journal (2003) 84: 3968–3981
- Malecki A, et al. 4-Hydroxynonenal Induces Oxidative Stress and Death of Cultured Spinal Cord Neurons. Journal of Neurochemistry (2000) 74: 2278-2287
- Arndt-Jovin DJ, et al. Multivariate Chromosome Analysis and Complete Karyotyping Using Dual Labeling and Fluorescence Digital Imaging Microscopy. Cytometry (1990) 11:80-93