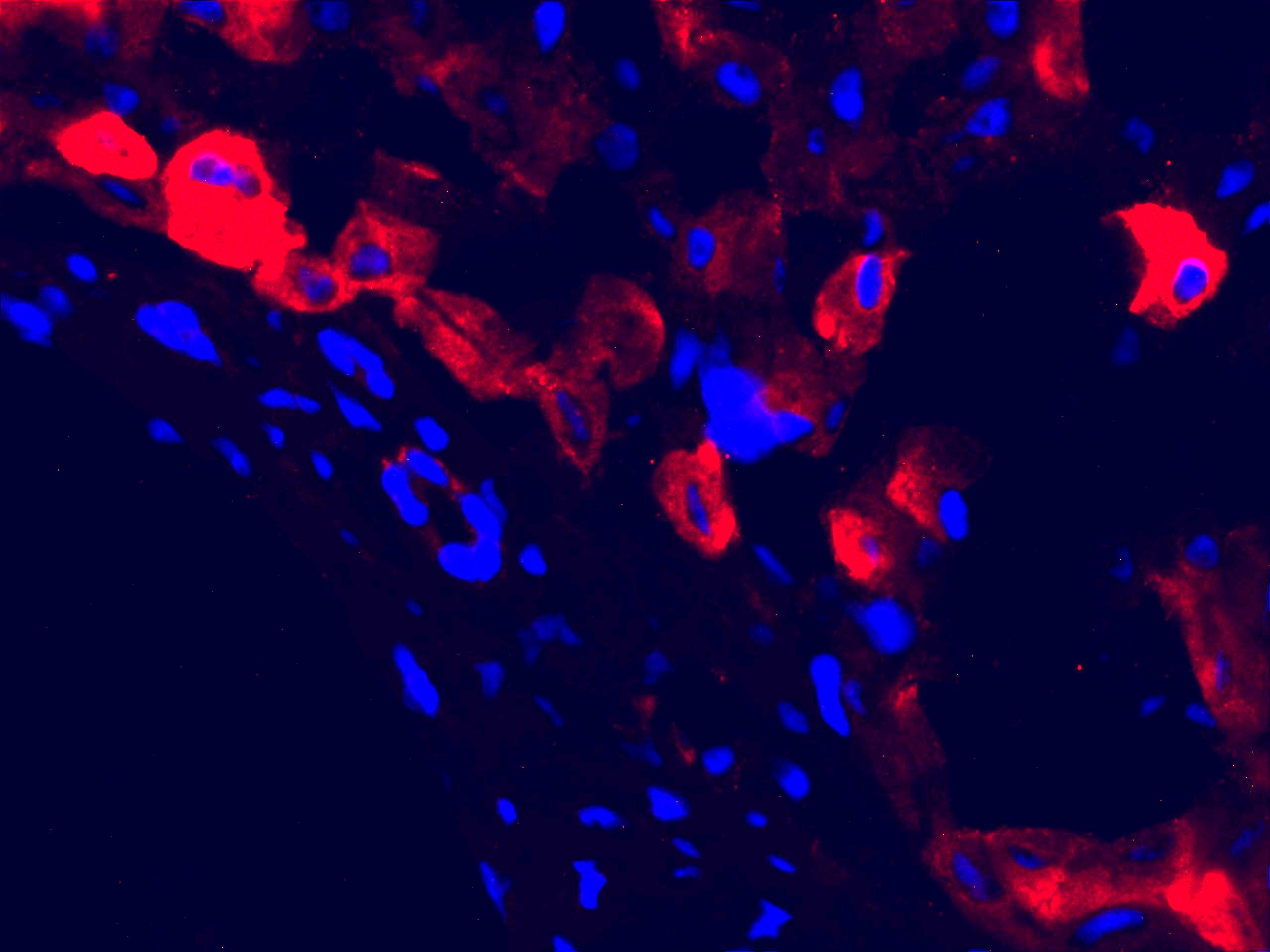
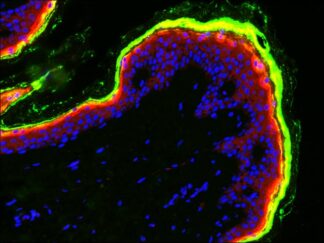
Description
Immunofluorescence (IF) is a vital immunochemical technique enabling the detection and precise localization of numerous antigens across diverse tissue types and various cell preparations. We are pleased to offer the VitroView™ immunofluorescence single staining Kit, a reliable and user-friendly solution designed for use with primary antibodies specifically raised in mice.
The advantages of this technology include: 1) High sensitivity; 2) Low background; 3) Reduction of steps and time; 4) Simplified multiple labeling.
Specifications
| Unit Size | 1 kit |
| Target Species | Mouse |
| Conjugates and Color of Fluorescence | FL594 Conjugated Goat anti Mouse IgG (Red) |
| Excitation/Emission for FL594 dye | 590/617 nm |
| Host Species | Goat |
| Application | Immunofluorescence staining for detecting a primary antibody made in mouse. |
Contents
- RTU Normal Goat Serum ——————————–10ml
- Goat anti Mouse FL594 (1mg/ml)———————–50µl
- Aqueous Anti-fade Mounting Medium with Dapi—- 1.5 ml×2
Note: RTU=ready-to-use
Reagents and Material Required but Not Provided
- Xylene and ethanol
- Distilled or deionized water
- 10 mM phosphate-buffered saline (PBS), pH 7.4
- Triton X-100
- Mini PAP Pen
- Primary antibody
- Antibody Dilution Buffer (SKU#: VB-6002)
- BSA
- Antigen retrieval reagents
Storage Condition
Store at 2-8°C.
Protocol
1. Preparation of Slides
A. Cell Lines
Grow cultured cells on sterile glass cover slips or slides overnight at 37°C. Briefly wash with PBS. Choose one of the following fixation methods (To achieve optimal results, it may be necessary to optimize the fixation methods):
- Fix in 10% formalin in PBS for 20 minutes (keep wet).
- Immerse in ice-cold methanol for 10 minutes and allow to air dry.
- immerse in ice-cold acetone for 10 minutes and allow to air dry.
Wash slides in PBS.
B. Frozen Sections
- Snap-freeze fresh tissues in liquid nitrogen or isopentane pre-cooled in liquid nitrogen, then embed them in OCT compound in cryomolds. Store the frozen tissue block at -80°C until ready for sectioning.
- Before sectioning, equilibrate the frozen tissue block to the temperature of the cryotome cryostat (e.g., -20°C).
- Section the frozen tissue block into the desired thickness (typically 5-10 µm) using the cryotome.
- Place the tissue sections onto glass slides suitable for immunohistochemistry (e.g., Superfrost).
- Store sections in a sealed slide box at -80°C for later use.
- Before staining, allow slides to warm to room temperature for 30 minutes and then fix in ice-cold acetone or ice-cold methanol for 10 minutes. Air dry for 30 minutes.
- Wash in PBS.
C. Paraffin Sections
- Deparaffinize sections in xylene, 3×5 minutes.
- Hydrate with 100% ethanol, 2×2 minutes.
- Hydrate with 95% ethanol, 2×2 minutes.
- Rinse in distilled water.
- Follow the required pretreatment procedure.
2. Antigen Retrieval
Most formalin-fixed tissues require antigen retrieval before proceeding with immunohistochemical staining. Common methods include heat-mediated and enzymatic antigen retrievals.
Choose the appropriate method:
- For Citrate: Bring slides to a boil in 10 mM sodium citrate buffer, pH 6.0, and maintain at a sub-boiling temperature for 10 minutes. Cool slides on the benchtop for 30 minutes.
- For EDTA: Bring slides to a boil in 1 mM EDTA, pH 8.0, and follow with 15 minutes at a sub-boiling temperature. No cooling is necessary.
- For TE: Bring slides to a boil in 10 mM TE/1 mM EDTA, pH 9.0, and then maintain at a sub-boiling temperature for 18 minutes. Cool at room temperature for 30 minutes.
- For Pepsin: Digest for 10 minutes at 37°C.
Note: Do not use this pretreatment with frozen sections or cultured cells that are not paraffin-embedded.
3. Staining Procedure
- Rinse sections in PBS-Triton X-100 (0.025%) for two 2-minute cycles.
- Serum Blocking: Incubate sections with 2-4 drops of ready-to-use (RTU) normal goat serum for 30 minutes to block non-specific immunoglobulin binding.
- Primary Antibody: Incubate sections with primary mouse antibody at the appropriate dilution in antibody dilution buffer (SKU# #: VB-6002) for 1-2 hours at room temperature or overnight at 2-8°C. Rinse in PBS.
- Detection: Incubate sections with FL594-conjugated Goat anti-Mouse IgG secondary antibody at suitable dilutions (ranging from 1/100 to 1/500) in 0.1% BSA in PBS for 1 hour at room temperature while keeping the samples in the dark.
- Rinse in PBS for three 2-minute cycles.
- Counterstaining and mount cover slip with 20-30µl of aqueous anti-fade mounting medium with DAPI. Seal cover slip with nail polish to prevent drying and movement.
- Visualize and capture the signal using a fluorescence microscope equipped with excitation wavelength filters of 594 nm.
- Store slides in the dark at 2-8°C.
More Images
User Manual and Material Safety Data Sheet (MSDS) (PDF)
Note:
This product is intended for research purposes only. This product is not intended to be used for therapeutic or diagnostic purposes in humans or animals.
Precautions:
Handle with care. Avoid contact with eyes, skin and clothing. Do not ingest. Wear gloves.