Description
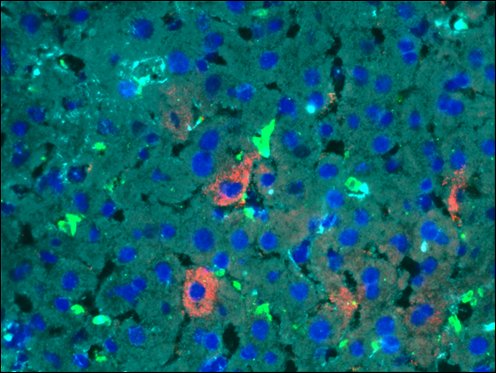
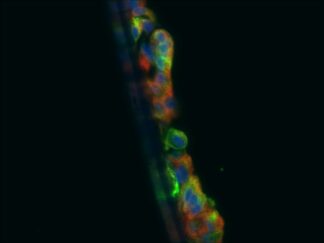
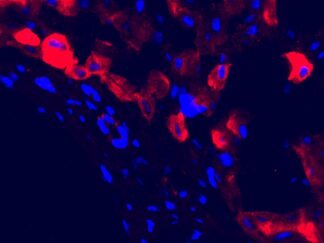
Triple or multiple immunofluorescence (IF) procedure is needed in order to be able to examine the co-distribution of three or more different antigens in the same sample. Here we provide reliable convenient VitroView™ Immunofluorescence Triple Staining Kit which can be carried out with 3 primary antibodies raised in the species of mouse, rabbit and rat.
The advantages of this technology include: 1) High sensitivity; 2) Low background; 3) Reduction of steps and time; 4) Simplified multiple labeling.
Specifications
| Unit Size | 1 kit |
| Target Species | Mouse, Rabbit and Rat |
| Conjugates and Color of Fluorescence | FL488 Conjugated Goat anti Mouse IgG (Green)
FL594 Conjugated Goat anti Rabbit IgG (Red) FL647 Conjugated Goat anti Rat IgG (Far Red) |
| Excitation/Emission for FL488 dye | 496/519 nm |
| Excitation/Emission for FL594 dye | 590/617 nm |
| Excitation/Emission for FL647 dye | 647/664 nm |
| Host Species | Goat |
| Application | Triple IF staining for simultaneous detection of primary antibodies raised in mouse, rabbit and rat |
Contents
- RTU Normal Goat Serum ————————————————-10ml
- FL488 Conjugated Goat anti Mouse (1mg/ml)——————50µl
- FL594 Conjugated Goat anti Rabbit (1mg/ml)——————50µl
- FL647 Conjugated Goat anti Rat (1mg/ml) ———————-50µl
- Aqueous Anti-fade Mounting Medium with Dapi———— 1.5 ml×2
Note: RTU=ready-to-use
Reagents and Material Required but Not Provided
-
- Xylene and ethanol
- Distilled or deionized water
- 10 mM phosphate-buffered saline (PBS), pH 7.4
- Triton X-100
- Mini PAP Pen
- Primary antibody
- Antibody Dilution Buffer (SKU#: VB-6002)
- BSA
Storage Condition
Store at 2-8°C.
Protocol
1. Preparation of Slides
A. Cell Lines
- Grow cultured cells on sterile glass cover slips or slides overnight at 37°C.
- Briefly wash with PBS.
- Choose one of the following fixation methods (To achieve optimal results, it may be necessary to optimize the fixation methods):
a. Fix in 10% formalin in PBS for 20 minutes (keep wet).
b. Immerse in ice-cold methanol for 10 minutes and allow to air dry.
c. immerse in ice-cold acetone for 10 minutes and allow to air dry.
- Wash slides in PBS.
B. Frozen Sections
- Snap-freeze fresh tissues in liquid nitrogen or isopentane pre-cooled in liquid nitrogen, then embed them in OCT compound in cryomolds. Store the frozen tissue block at -80°C until ready for sectioning.
- Before sectioning, equilibrate the frozen tissue block to the temperature of the cryotome cryostat (e.g., -20°C).
- Section the frozen tissue block into the desired thickness (typically 5-10 µm) using the cryotome.
- Place the tissue sections onto glass slides suitable for immunohistochemistry (e.g., Superfrost).
- Store sections in a sealed slide box at -80°C for later use.
- Before staining, allow slides to warm to room temperature for 30 minutes and then fix in ice-cold acetone or ice-cold methanol for 10 minutes. Air dry for 30 minutes.
- Wash in PBS.
C. Paraffin Sections
- Deparaffinize sections in xylene, 3×5 minutes.
- Hydrate with 100% ethanol, 2×2 minutes.
- Hydrate with 95% ethanol, 2×2 minutes.
- Rinse in distilled water.
- Follow the required pretreatment procedure.
2. Antigen Retrieval
- Most formalin-fixed tissues require antigen retrieval before proceeding with immunohistochemical staining. Common methods include heat-mediated and enzymatic antigen retrievals.
- Choose the appropriate method:
a. For Citrate: Bring slides to a boil in 10 mM sodium citrate buffer, pH 6.0, and maintain at a sub-boiling temperature for 10 minutes. Cool slides on the benchtop for 30 minutes.
b. For EDTA: Bring slides to a boil in 1 mM EDTA, pH 8.0, and follow with 15 minutes at a sub-boiling temperature. No cooling is necessary.
c. For TE: Bring slides to a boil in 10 mM TE/1 mM EDTA, pH 9.0, and then maintain at a sub-boiling temperature for 18 minutes. Cool at room temperature for 30 minutes.
d. For Pepsin: Digest for 10 minutes at 37°C.
Note: Do not use this pretreatment with frozen sections or cultured cells that are not paraffin-embedded.
3. Staining Procedure
- Rinse sections in PBS-Triton X-100 (0.025%) for two 2-minute cycles.
- Serum Blocking: Incubate sections with 2-4 drops of ready-to-use (RTU) normal goat serum for 30 minutes to block non-specific immunoglobulin binding.
- Primary Antibody: Incubate sections with a mixture of three primary antibodies (mouse, rabbit and rat) at the appropriate dilution in antibody dilution buffer (SKU# #: VB-6002) for 1-2 hours at room temperature or overnight at 4°C. Rinse in PBS.
- Detection: Incubate sections with a mixture (70-150 µl) of three secondary antibodies raised in different species (with three fluorochromes including FL488 conjugated anti-mouse / FL594 conjugated anti-rabbit /FL647 conjugated anti rat secondary antibodies) at appropriate dilutions (1/100-1/500) in 0.1% BSA in PBS for 1 hour at room temperature in the dark.
- Rinse in PBS for three 2-minute cycles.
- Counterstaining and mount cover slip with 20-30µl of aqueous anti-fade mounting medium with DAPI. Seal cover slip with nail polish to prevent drying and movement.
- Visualize and capture the signal using a fluorescence microscope equipped with excitation wavelength filters of 488 nm, 594 nm and 647nm.
- Store slides in the dark at 4°C.