Routine Histology Services – Expert Tissue Processing & Staining
Routine histology services are essential for tissue-based research and diagnostics, enabling researchers and pathologists to analyze tissue morphology, identify specific cell types, and detect microorganisms like bacteria. Histological staining enhances tissue visualization by adding contrast to otherwise transparent tissue sections.
Types of Histology Staining Services
1. Routine Staining (Hematoxylin & Eosin – H&E Stain)
- The H&E stain is the standard technique used for all tissue specimens, revealing structural details and underlying tissue conditions.
2. Special Stains
- Special stains provide additional insights beyond H&E, highlighting specific cellular components, pathogens, or tissue abnormalities.
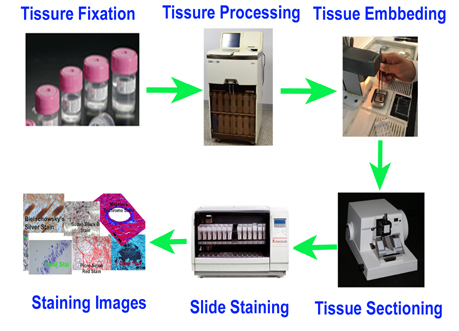
Comprehensive Routine Histology Services
We offer a full range of histology lab services, from tissue processing to high-quality staining:
✅ Tissue Trimming – Precision trimming for optimal sectioning.
✅ Tissue Processing – Formalin fixation and paraffin embedding (FFPE).
✅ Paraffin Embedding – Long-term preservation of tissue samples.
✅ Tissue Sectioning – Thin section cutting from paraffin-embedded blocks.
✅ Slide Preparation – Mounting on positively charged or custom-treated slides.
✅ H&E & Special Staining – High-quality staining for enhanced tissue visualization.
🔬 Get Expert Routine Histology Services Today! Contact us for high-quality tissue processing, embedding, and staining solutions tailored to your research needs.
FFPE Histology Service Overview Video
Frequently Asked Questions (FAQ)
FAQ 1. How do I prepare the tissue samples for tissue processing?
- The usual fixative for paraffin embedded tissues is neutral buffered formalin (NBF). This is equivalent to 4% fresh paraformaldehyde in a buffered solution
- Where the best possible morphology is required, animals should be anesthesized and subjected to cardiac perfusion with saline, followed by a 10% formalin flush. If biochemical studies need to be performed on the tissue, a 10% formalin flush should not be used as it may interfere with subsequent analysis.
- For routine stains where perfusion is not required, tissue is sectioned and drop-fixed in a 10% formalin solution. Fixative volume should be 20 times that of tissue on a weight per volume; use 2 ml of formalin per 100 mg of tissue.
- Due to the slow rate of diffusion of formalin (0.5 mm/hr), tissue should be sectioned into 3-5 mm slices before transferring into formalin. This will ensure the best possible preservation of tissue and offers rapid uniform penetration and fixation of tissue within 3 hours.Tissue should be fixed for a minimum 48 hours at room temperature.
- After 48 hours of fixation, move tissue into 70% ethanol for long term storage.
FAQ 2. What is Hematoxylin and Eosin (H&E) stain?
Hematoxylin and Eosin (H&E) stain is the most commonly used staining system. It is an important part of VitroVivo routine histology services. H&E contains two dyes haemotoxylin and eosin. Eosin is an acidic dye: it is negatively charged (general formula for acidic dyes is: Na+dye-). It stains basic (or acidophilic) structures red or pink. This is also sometimes termed ‘eosinophilic’. Thus the cytoplasm is stained pink by H&E staining.
Hematoxylin can be considered as a basic dye (general formula for basic dyes is:dye+ Cl-). Hematoxylin is actually a dye called hematein (obtained from the log-wood tree) used in combination with aluminium ions (Al3+). It is used to stain acidic (or basophilic) structures a purplish blue. (Hematoxylin is not strictly a basic dye, but it is used with a ‘mordant’ that makes this stain act as a basic dye. The mordant (aluminium salts) binds to the tissue, and then hematoxylin binds to the mordant, forming a tissue-mordant-hematoxylin linkage.) Thus the nucleus is stained purple by H&E staining.
This means that the nucleus, and parts of the cytoplasm that contain RNA stain up in one color (purple), and the rest of the cytoplasm stains up a different color (pink)

