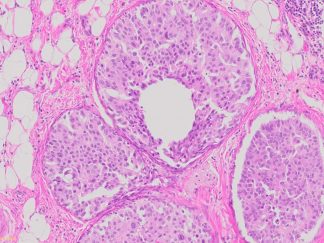
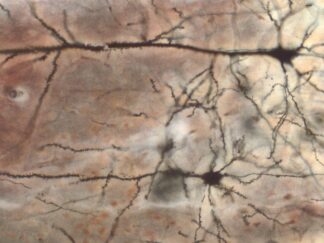
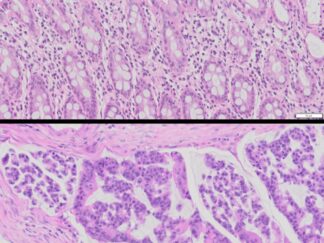
Description
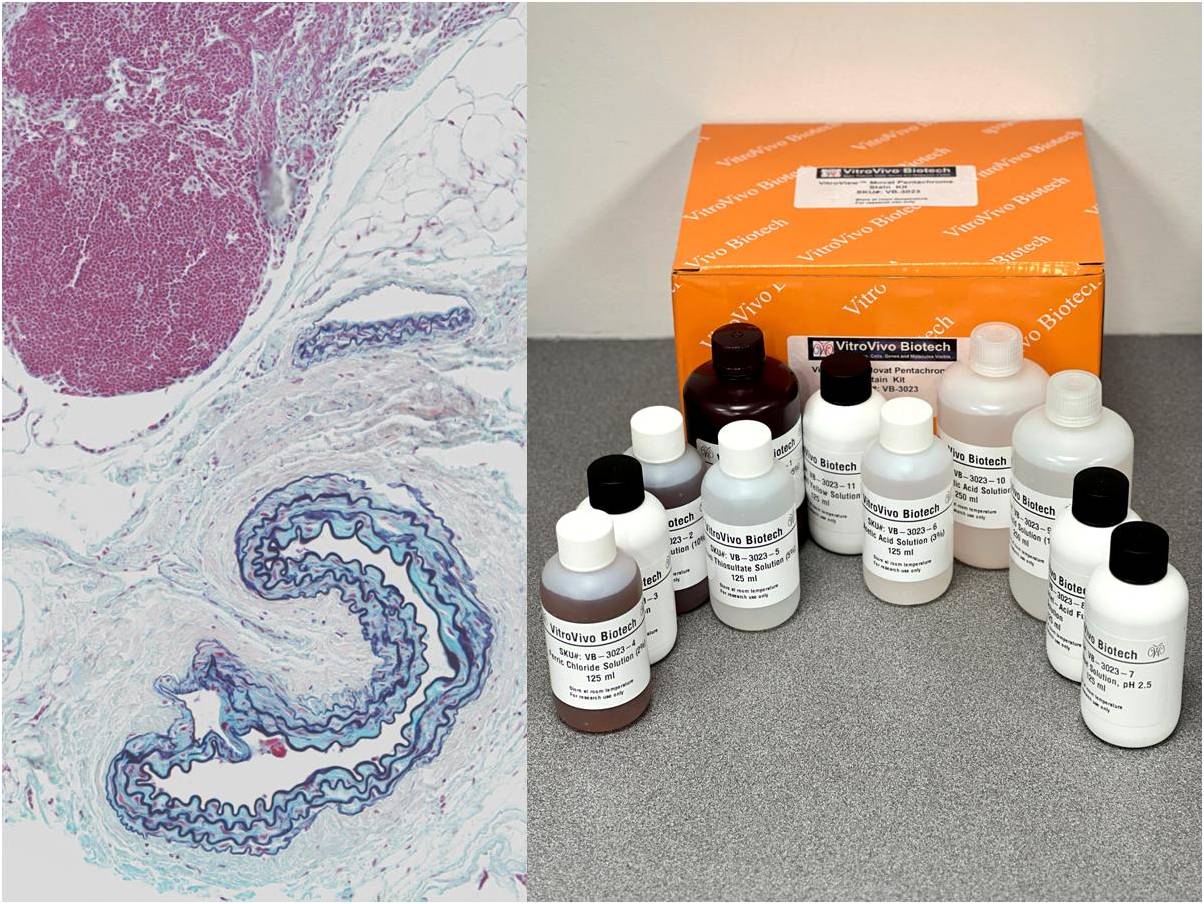
The VitroView™ Movat Pentachrome Stain Kit (Modified Russel-Movat ) is intended for use in histological visualization of collagen, muscle tissue, reticular fibers, mucins and fibrin in tissue sections. This kit is a very useful tool to study the heart, blood vessels and various vascular diseases.
Kit Components
| SKU# | Reagent Name | Volume (ml) |
| VB-3023-1 | Alcoholic Hematoxylin (5%) | 250 |
| VB-3023-2 | Ferric Chloride Solution (10%) | 125 |
| VB-3023-3 | Iodine Solution | 125 |
| VB-3023-4 | Ferric Chloride Solution (2%) | 125 |
| VB-3023-5 | Sodium Thiosulfate Solution (5%) | 125 |
| VB-3023-6 | Acetic Acid Solution (3%) | 125 |
| VB-3023-7 | Alcian Blue Solution, pH 2.5 | 125 |
| VB-3023-8 | Biebrich Scarlet–Acid Fuchsin Solution | 125 |
| VB-3023-9 | Acetic Acid Solution (1%) | 250 |
| VB-3023-10 | Phosphotungstic Acid Solution (5%) | 250 |
| VB-3023-11 | Metanil Yellow Solution | 125 |
Storage
Room temperature.
Protocol
Things to do before starting the procedures:
Prepare a working Hematoxylin Solution by mixing 30 ml of Hematoxylin (5%) Solution, 15 ml of Ferric Chloride Solution (10%) and 15 mL of Iodine Solution. Note: Mixed solution may be used for 24 hours.
Procedures for FFPE tissue sections
- Deparaffinize in Xylene I for 6 minutes and II for 6 minutes.
- Rehydrate: Ethanol 100% (2 minutes); Ethanol 100% (2 minutes); Ethanol 95% (2 minutes); Ethanol 95% (2 minutes); Ethanol 70% (2 minutes).
- Rinse in distilled water (5 minutes).
- Stain tissue section with working Hematoxylin Solution for 20 minutes.
- Rinse in running tap water until no excess stain remains on slide.
- Dip slide in Ferric Chloride Solution (2%) 15-20 times and rinse in tap water
- Check slides microscopically for proper differentiation. Repeat step 3-6 if required.
- Rinse in 2 changes of distilled water.
- Place slide in Sodium Thiosulfate Solution (5%) and incubate for 1 minute.
- Rinse in tap water for 2 minutes followed by 2 changes in distilled water.
- Place slide in Acetic Acid Solution (3%) and incubate for 2 minutes to equilibrate tissue prior to staining with Alcian Blue Solution, pH 2.5.
- Without rinsing, place slide in Alcian Blue Solution, pH 2.5 and incubate for 25 minutes. Rinse in tap water for 2 minutes followed by 2 changes in distilled water.
- Place slide in Biebrich Scarlet–Acid Fuchsin Solution and incubate for 2 minutes.
- Rinse slide in 2 changes of distilled water.
- Place slide in Acetic Acid Solution (1%) for 5-10 seconds with agitation.
- Rinse quickly in distilled water.
- Differentiate slide in 2 changes of Phosphotungstic Acid Solution (5%) for 3-7 minutes each.
- Check slides microscopically for proper differentiation.
- Collagen should be clear but elastic fibers should still be stained. Repeat step 17 if required.
- Rinse slide briefly in distilled water.
- Dip slide several times (3-5) in Acetic Acid Solution (1%).
- Shake off excess Acetic Acid Solution (1%) and without rinsing apply Metanil Yellow Solution and incubate for 10 minutes.
- Rinse slide in 2 changes of absolute alcohol (1 min per change)
- Clear with 2 changes of xylene (5 minutes per change) and coverslip with Permount or other suitable organic mounting medium.
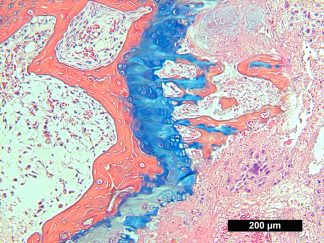
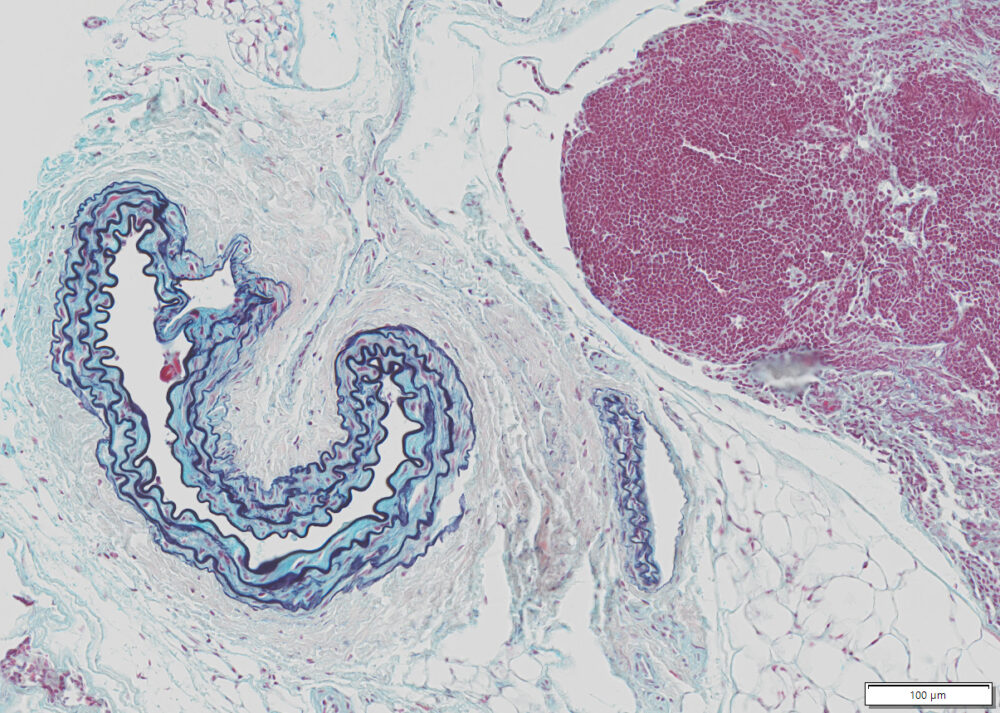
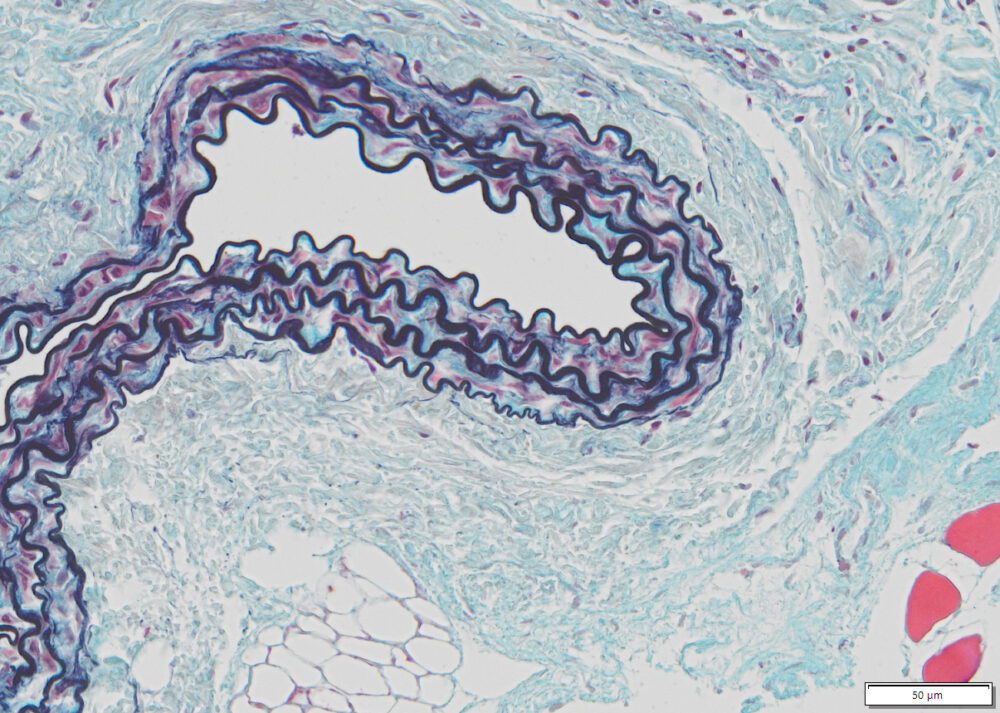
Expected results
| Nuclei and Elastic Fibers | Black to blue black |
| Ground Substance and Mucin | Bright blue |
| Muscle | Red |
| Collagen | Yellow to red |
| Reticular Fibers | Yellow |
| Fibrinioid and Fibrin | Intense Red |
Control Tissue
Lung, skin, colon, heart or any vascular tissue.
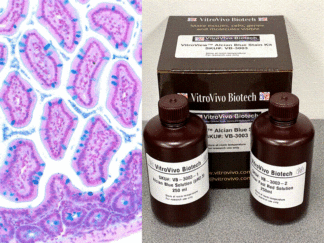
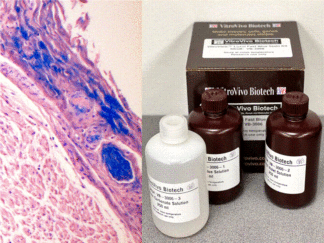
More Images
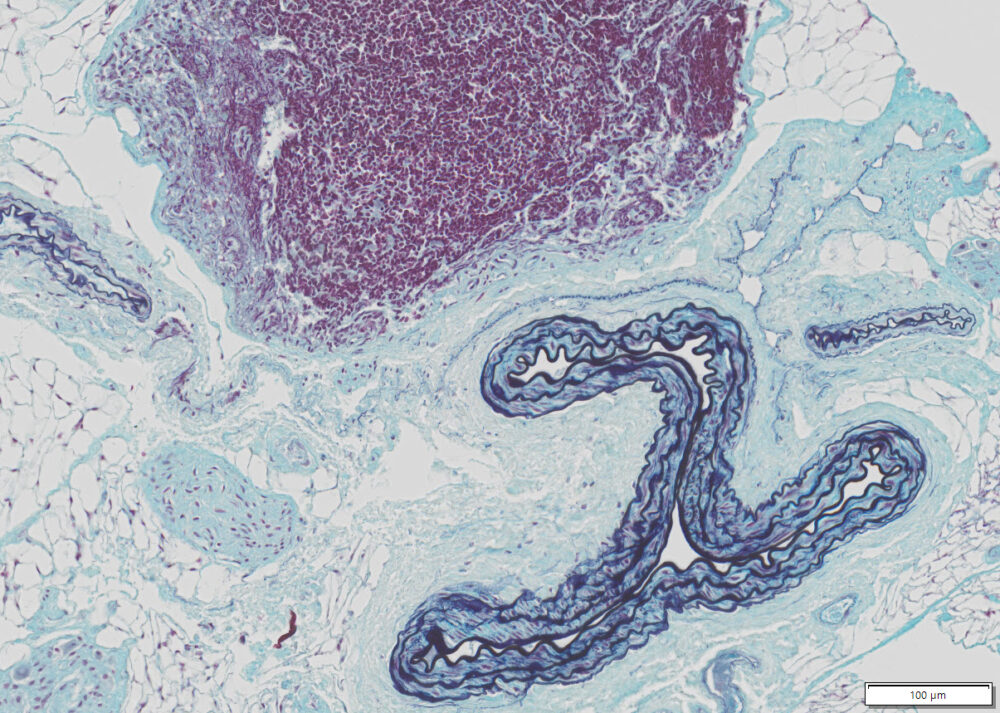
Note
This product is intended for research purposes only. This product is not intended to be used for therapeutic or diagnostic purposes in humans or animals.
Precautions
Handle with care. Avoid contact with eyes, skin and clothing. Do not ingest. Wear gloves.